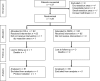
Randomized controlled multicenter trial on the effectiveness of the collagen hemostat Sangustop® compared with a carrier-bound fibrin sealant during liver resection (ESSCALIVER study, NCT00918619)
- PMID: 24880345
- PMCID: PMC4099526
- DOI: 10.1007/s00423-014-1203-9
Randomized controlled multicenter trial on the effectiveness of the collagen hemostat Sangustop® compared with a carrier-bound fibrin sealant during liver resection (ESSCALIVER study, NCT00918619)
Abstract
Background: Despite improvements in liver surgery over the past decades, hemostasis during hepatic resections remains challenging. This multicenter randomized study compares the hemostatic effect of a collagen hemostat vs. a carrier-bound fibrin sealant after hepatic resection.
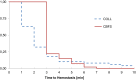
Methods: Patients scheduled for elective liver resection were randomized intraoperatively to receive either the collagen hemostat (COLL) or the carrier-bound fibrin sealant (CBFS) for secondary hemostasis. The primary endpoint was the proportion of patients with hemostasis after 3 min. Secondary parameters were the proportions of patients with hemostasis after 5 and 10 min, the total time to hemostasis, and the complication rates during a 3 months follow-up period.
Results: A total of 128 patients were included. In the COLL group, 53 out of 61 patients (86.9 %) achieved complete hemostasis within 3 min after application of the hemostat compared to 52 out of 65 patients (80.0 %) in the CBFS group. The 95 % confidence interval for this difference [-6.0 %, 19.8 %] does not include the lower noninferiority margin (-10 %). Thus, the COLL treatment can be regarded as noninferior to the comparator. The proportions of patients with hemostasis after 3, 5, and 10 min were not significantly different between the two study arms. Postoperative mortality and morbidity were similar in both treatment groups.
Conclusion: The collagen hemostat is as effective as the carrier-bound fibrin sealant in obtaining secondary hemostasis during liver resection with a comparable complication rate.
Trial registration: ClinicalTrials.gov NCT00918619.
Figures
Similar articles
-
Comparison of the collagen haemostat Sangustop® versus a carrier-bound fibrin sealant during liver resection; ESSCALIVER-Study.Trials. 2010 Nov 19;11:109. doi: 10.1186/1745-6215-11-109. Trials. 2010. PMID: 21087530 Free PMC article. Clinical Trial.
-
Results of a randomised controlled trial between an ORC collagen hemostatic agent and a carrier-bound fibrin sealant.J Visc Surg. 2021 Feb;158(1):11-18. doi: 10.1016/j.jviscsurg.2020.06.015. Epub 2020 Jul 31. J Visc Surg. 2021. PMID: 32747306 Clinical Trial.
-
Evaluation of a novel hemostatic device in an ovine parenchymal organ bleeding model of normal and impaired hemostasis.J Biomed Mater Res. 2002;63(1):37-47. doi: 10.1002/jbm.10081. J Biomed Mater Res. 2002. PMID: 11787027 Clinical Trial.
-
Role of fibrin sealants in liver surgery.Dig Surg. 2012;29(1):54-61. doi: 10.1159/000335735. Epub 2012 Mar 15. Dig Surg. 2012. PMID: 22441621 Review.
-
Use of fibrin-based sealants and gelatin-matrix hemostats in laparoscopic liver surgery.Surg Laparosc Endosc Percutan Tech. 2011 Jun;21(3):131-41. doi: 10.1097/SLE.0b013e31821db688. Surg Laparosc Endosc Percutan Tech. 2011. PMID: 21654294 Review.
Cited by
-
Methods to decrease blood loss during liver resection: a network meta-analysis.Cochrane Database Syst Rev. 2016 Oct 31;10(10):CD010683. doi: 10.1002/14651858.CD010683.pub3. Cochrane Database Syst Rev. 2016. PMID: 27797116 Free PMC article.
-
Health and Economic Advantages Associated With the Use of TachoSil: An Update of Systematic Review.Ther Clin Risk Manag. 2025 Mar 1;21:257-271. doi: 10.2147/TCRM.S476650. eCollection 2025. Ther Clin Risk Manag. 2025. PMID: 40046260 Free PMC article.
-
SPOT GRADE II: Clinical Validation of a New Method for Reproducibly Quantifying Surgical Wound Bleeding: Prospective, Multicenter, Multispecialty, Single-Arm Study.Clin Appl Thromb Hemost. 2020 Jan-Dec;26:1076029620936340. doi: 10.1177/1076029620936340. Clin Appl Thromb Hemost. 2020. PMID: 32703005 Free PMC article.
-
A Prospective, Randomized, Phase III Study to Evaluate the Efficacy and Safety of Fibrin Sealant Grifols as an Adjunct to Hemostasis as Compared to Cellulose Sheets in Hepatic Surgery Resections.J Gastrointest Surg. 2018 Nov;22(11):1939-1949. doi: 10.1007/s11605-018-3852-4. Epub 2018 Jul 2. J Gastrointest Surg. 2018. PMID: 29967969 Clinical Trial.
-
NHS-POx-loaded patch versus fibrin sealant patch in a porcine robotic liver bleeding model.BMC Surg. 2023 Aug 28;23(1):257. doi: 10.1186/s12893-023-02159-4. BMC Surg. 2023. PMID: 37641071 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous