CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype
- PMID: 24880458
- PMCID: PMC4105703
- DOI: 10.1038/ni.2914
CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype
Abstract
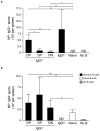
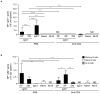
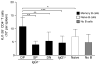
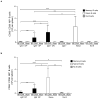
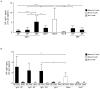
Memory B cells (MBCs) are long-lived sources of rapid, isotype-switched secondary antibody-forming cell (AFC) responses. Whether MBCs homogeneously retain the ability to self-renew and terminally differentiate or if these functions are compartmentalized into MBC subsets has remained unclear. It has been suggested that antibody isotype controls MBC differentiation upon restimulation. Here we demonstrate that subcategorizing MBCs on the basis of their expression of CD80 and PD-L2, independently of isotype, identified MBC subsets with distinct functions upon rechallenge. CD80(+)PD-L2(+) MBCs differentiated rapidly into AFCs but did not generate germinal centers (GCs); conversely, CD80(-)PD-L2(-) MBCs generated few early AFCs but robustly seeded GCs. The gene-expression patterns of the subsets supported both the identity and function of these distinct MBC types. Hence, the differentiation and regeneration of MBCs are compartmentalized.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
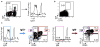
Similar articles
-
Transiently antigen primed B cells can generate multiple subsets of memory cells.PLoS One. 2017 Aug 29;12(8):e0183877. doi: 10.1371/journal.pone.0183877. eCollection 2017. PLoS One. 2017. PMID: 28850584 Free PMC article.
-
IgM+ and IgM- memory B cells represent heterogeneous populations capable of producing class-switched antibodies and germinal center B cells upon rechallenge with P. yoelii.J Leukoc Biol. 2022 Nov;112(5):1115-1135. doi: 10.1002/JLB.4A0921-523R. Epub 2022 Jun 3. J Leukoc Biol. 2022. PMID: 35657097 Free PMC article.
-
B Cell Subsets Differentially Contribute to the T Cell-Independent Memory Pool.J Immunol. 2020 Nov 1;205(9):2362-2374. doi: 10.4049/jimmunol.1901453. Epub 2020 Sep 25. J Immunol. 2020. PMID: 32978280 Free PMC article.
-
Memory B cell heterogeneity: Remembrance of things past.J Leukoc Biol. 2018 Feb;103(2):269-274. doi: 10.1002/JLB.4MR0517-215R. Epub 2018 Jan 17. J Leukoc Biol. 2018. PMID: 29345369 Free PMC article. Review.
-
Memory B Cells of Mice and Humans.Annu Rev Immunol. 2017 Apr 26;35:255-284. doi: 10.1146/annurev-immunol-041015-055531. Epub 2017 Jan 30. Annu Rev Immunol. 2017. PMID: 28142324 Review.
Cited by
-
Stimulation history is epigenetically imprinted in memory B cells.Nat Immunol. 2024 Aug;25(8):1328-1329. doi: 10.1038/s41590-024-01903-z. Nat Immunol. 2024. PMID: 38982287 No abstract available.
-
Somatically Hypermutated Plasmodium-Specific IgM(+) Memory B Cells Are Rapid, Plastic, Early Responders upon Malaria Rechallenge.Immunity. 2016 Aug 16;45(2):402-14. doi: 10.1016/j.immuni.2016.06.014. Epub 2016 Jul 26. Immunity. 2016. PMID: 27473412 Free PMC article.
-
The whole-cell pertussis vaccine imposes a broad effector B cell response in mouse heterologous prime-boost settings.JCI Insight. 2022 Nov 8;7(21):e157034. doi: 10.1172/jci.insight.157034. JCI Insight. 2022. PMID: 36136586 Free PMC article.
-
Antibody Responses to SARS-CoV-2: Let's Stick to Known Knowns.J Immunol. 2020 Nov 1;205(9):2342-2350. doi: 10.4049/jimmunol.2000839. Epub 2020 Sep 4. J Immunol. 2020. PMID: 32887754 Free PMC article. Review.
-
Advances in understanding the formation and fate of B-cell memory in response to immunization or infection.Oxf Open Immunol. 2021 Sep 11;2(1):iqab018. doi: 10.1093/oxfimm/iqab018. eCollection 2021. Oxf Open Immunol. 2021. PMID: 36845573 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

