Regulation of autophagy by amino acids and MTOR-dependent signal transduction
- PMID: 24880909
- PMCID: PMC4580722
- DOI: 10.1007/s00726-014-1765-4
Regulation of autophagy by amino acids and MTOR-dependent signal transduction
Abstract
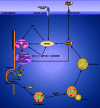
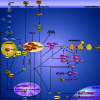
Amino acids not only participate in intermediary metabolism but also stimulate insulin-mechanistic target of rapamycin (MTOR)-mediated signal transduction which controls the major metabolic pathways. Among these is the pathway of autophagy which takes care of the degradation of long-lived proteins and of the elimination of damaged or functionally redundant organelles. Proper functioning of this process is essential for cell survival. Dysregulation of autophagy has been implicated in the etiology of several pathologies. The history of the studies on the interrelationship between amino acids, MTOR signaling and autophagy is the subject of this review. The mechanisms responsible for the stimulation of MTOR-mediated signaling, and the inhibition of autophagy, by amino acids have been studied intensively in the past but are still not completely clarified. Recent developments in this field are discussed.
Keywords: Glutamine; Leucine; Lysosomes; Mitochondria; Rapamycin.
Figures
References
-
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. - DOI - PMC - PubMed
-
- Albracht SP, Meijer AJ, Rydstrom J. Mammalian NADH:ubiquinone oxidoreductase (Complex I) and nicotinamide nucleotide transhydrogenase (Nnt) together regulate the mitochondrial production of H(2)O(2)–implications for their role in disease, especially cancer. J Bioenerg Biomembr. 2011;43:541–564. doi: 10.1007/s10863-011-9381-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

