Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies
- PMID: 24881045
- PMCID: PMC4258786
- DOI: 10.1093/ije/dyu115
Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies
Abstract
Background: Blinding patients in clinical trials is a key methodological procedure, but the expected degree of bias due to nonblinded patients on estimated treatment effects is unknown.
Methods: Systematic review of randomized clinical trials with one sub-study (i.e. experimental vs control) involving blinded patients and another, otherwise identical, sub-study involving nonblinded patients. Within each trial, we compared the difference in effect sizes (i.e. standardized mean differences) between the sub-studies. A difference <0 indicates that nonblinded patients generated a more optimistic effect estimate. We pooled the differences with random-effects inverse variance meta-analysis, and explored reasons for heterogeneity.
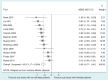
Results: Our main analysis included 12 trials (3869 patients). The average difference in effect size for patient-reported outcomes was -0.56 (95% confidence interval -0.71 to -0.41), (I(2)=60%, P=0.004), i.e. nonblinded patients exaggerated the effect size by an average of 0.56 standard deviation, but with considerable variation. Two of the 12 trials also used observer-reported outcomes, showing no indication of exaggerated effects due lack of patient blinding. There was a larger effect size difference in 10 acupuncture trials [-0.63 (-0.77 to -0.49)], than in the two non-acupuncture trials [-0.17 (-0.41 to 0.07)]. Lack of patient blinding also increased attrition and use of co-interventions: ratio of control group attrition risk 1.79 (1.18 to 2.70), and ratio of control group co-intervention risk 1.55 (0.99 to 2.43).
Conclusions: This study provides empirical evidence of pronounced bias due to lack of patient blinding in complementary/alternative randomized clinical trials with patient-reported outcomes.
Keywords: Bias; blinding; patient blinding; randomized clinical trials; systematic review.
© The Author 2014; all rights reserved. Published by Oxford University Press on behalf of the International Epidemiological Association.
Figures
References
-
- Kaptchuk TJ. Intentional ignorance: a history of blind assessment and placebo controls in medicine. Bull Hist Med 1998;72:389–433. - PubMed
-
- Hróbjartsson A, Boutron I. Blinding in randomized clinical trials: imposed impartiality . Clin Pharmacol Ther 2011;90:732–36. - PubMed
-
- Hróbjartsson A, Thomsen ASS, Emanuelsson F, et al. Observer bias in randomized clinical trials with binary outcomes: systematic review of trials with both blinded and nonblinded outcome assessors. BMJ 2012;344:e1119. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

