Adjuvant exemestane with ovarian suppression in premenopausal breast cancer
- PMID: 24881463
- PMCID: PMC4175521
- DOI: 10.1056/NEJMoa1404037
Adjuvant exemestane with ovarian suppression in premenopausal breast cancer
Abstract
Background: Adjuvant therapy with an aromatase inhibitor improves outcomes, as compared with tamoxifen, in postmenopausal women with hormone-receptor-positive breast cancer.
Methods: In two phase 3 trials, we randomly assigned premenopausal women with hormone-receptor-positive early breast cancer to the aromatase inhibitor exemestane plus ovarian suppression or tamoxifen plus ovarian suppression for a period of 5 years. Suppression of ovarian estrogen production was achieved with the use of the gonadotropin-releasing-hormone agonist triptorelin, oophorectomy, or ovarian irradiation. The primary analysis combined data from 4690 patients in the two trials.
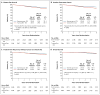
Results: After a median follow-up of 68 months, disease-free survival at 5 years was 91.1% in the exemestane-ovarian suppression group and 87.3% in the tamoxifen-ovarian suppression group (hazard ratio for disease recurrence, second invasive cancer, or death, 0.72; 95% confidence interval [CI], 0.60 to 0.85; P<0.001). The rate of freedom from breast cancer at 5 years was 92.8% in the exemestane-ovarian suppression group, as compared with 88.8% in the tamoxifen-ovarian suppression group (hazard ratio for recurrence, 0.66; 95% CI, 0.55 to 0.80; P<0.001). With 194 deaths (4.1% of the patients), overall survival did not differ significantly between the two groups (hazard ratio for death in the exemestane-ovarian suppression group, 1.14; 95% CI, 0.86 to 1.51; P=0.37). Selected adverse events of grade 3 or 4 were reported for 30.6% of the patients in the exemestane-ovarian suppression group and 29.4% of those in the tamoxifen-ovarian suppression group, with profiles similar to those for postmenopausal women.
Conclusions: In premenopausal women with hormone-receptor-positive early breast cancer, adjuvant treatment with exemestane plus ovarian suppression, as compared with tamoxifen plus ovarian suppression, significantly reduced recurrence. (Funded by Pfizer and others; TEXT and SOFT ClinicalTrials.gov numbers, NCT00066703 and NCT00066690, respectively.).
Figures

Comment in
-
Hormone therapy in premenopausal women with early-stage breast cancer.N Engl J Med. 2014 Jul 10;371(2):175-6. doi: 10.1056/NEJMe1405746. N Engl J Med. 2014. PMID: 25006724 No abstract available.
-
Exemestane with ovarian suppression in premenopausal breast cancer.N Engl J Med. 2014 Oct 2;371(14):1358-9. doi: 10.1056/NEJMc1409366. N Engl J Med. 2014. PMID: 25271610 No abstract available.
-
Exemestane with ovarian suppression in premenopausal breast cancer.N Engl J Med. 2014 Oct 2;371(14):1357. doi: 10.1056/NEJMc1409366. N Engl J Med. 2014. PMID: 25271611 No abstract available.
-
Exemestane with ovarian suppression in premenopausal breast cancer.N Engl J Med. 2014 Oct 2;371(14):1358. doi: 10.1056/NEJMc1409366. N Engl J Med. 2014. PMID: 25271612 No abstract available.
-
Exemestane with ovarian suppression in premenopausal breast cancer.N Engl J Med. 2014 Oct 2;371(14):1358. doi: 10.1056/NEJMc1409366. N Engl J Med. 2014. PMID: 25271613 No abstract available.
-
Perfecting breast-cancer treatment--incremental gains and musculoskeletal pains.N Engl J Med. 2015 Jan 29;372(5):477-8. doi: 10.1056/NEJMe1413164. Epub 2014 Dec 11. N Engl J Med. 2015. PMID: 25495491 No abstract available.
References
-
- Carlson RW, Allred DC, Anderson BO, et al. Breast cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–92. - PubMed
-
- Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
