Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia
- PMID: 24881631
- PMCID: PMC4134521
- DOI: 10.1056/NEJMoa1400376
Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia
Abstract
Background: In patients with chronic lymphoid leukemia (CLL) or small lymphocytic lymphoma (SLL), a short duration of response to therapy or adverse cytogenetic abnormalities are associated with a poor outcome. We evaluated the efficacy of ibrutinib, a covalent inhibitor of Bruton's tyrosine kinase, in patients at risk for a poor outcome.
Methods: In this multicenter, open-label, phase 3 study, we randomly assigned 391 patients with relapsed or refractory CLL or SLL to receive daily ibrutinib or the anti-CD20 antibody ofatumumab. The primary end point was the duration of progression-free survival, with the duration of overall survival and the overall response rate as secondary end points.
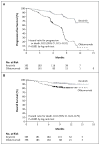
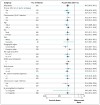
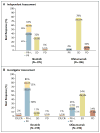
Results: At a median follow-up of 9.4 months, ibrutinib significantly improved progression-free survival; the median duration was not reached in the ibrutinib group (with a rate of progression-free survival of 88% at 6 months), as compared with a median of 8.1 months in the ofatumumab group (hazard ratio for progression or death in the ibrutinib group, 0.22; P<0.001). Ibrutinib also significantly improved overall survival (hazard ratio for death, 0.43; P=0.005). At 12 months, the overall survival rate was 90% in the ibrutinib group and 81% in the ofatumumab group. The overall response rate was significantly higher in the ibrutinib group than in the ofatumumab group (42.6% vs. 4.1%, P<0.001). An additional 20% of ibrutinib-treated patients had a partial response with lymphocytosis. Similar effects were observed regardless of whether patients had a chromosome 17p13.1 deletion or resistance to purine analogues. The most frequent nonhematologic adverse events were diarrhea, fatigue, pyrexia, and nausea in the ibrutinib group and fatigue, infusion-related reactions, and cough in the ofatumumab group.
Conclusions: Ibrutinib, as compared with ofatumumab, significantly improved progression-free survival, overall survival, and response rate among patients with previously treated CLL or SLL. (Funded by Pharmacyclics and Janssen; RESONATE ClinicalTrials.gov number, NCT01578707.).
Figures
Comment in
-
Targeted therapies: ibrutinib resonates with us.Nat Rev Clin Oncol. 2014 Jul;11(7):380. doi: 10.1038/nrclinonc.2014.105. Epub 2014 Jun 17. Nat Rev Clin Oncol. 2014. PMID: 24935013 No abstract available.
-
Changes in the treatment landscape for chronic lymphoid leukemia.N Engl J Med. 2014 Jul 17;371(3):273-4. doi: 10.1056/NEJMe1405766. N Engl J Med. 2014. PMID: 25014693 No abstract available.
References
-
- Hallek M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am J Hematol. 2013;88:803–16. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. - PubMed
-
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10. - PubMed
-
- Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
