Enzalutamide in metastatic prostate cancer before chemotherapy
- PMID: 24881730
- PMCID: PMC4418931
- DOI: 10.1056/NEJMoa1405095
Enzalutamide in metastatic prostate cancer before chemotherapy
Abstract
Background: Enzalutamide is an oral androgen-receptor inhibitor that prolongs survival in men with metastatic castration-resistant prostate cancer in whom the disease has progressed after chemotherapy. New treatment options are needed for patients with metastatic prostate cancer who have not received chemotherapy, in whom the disease has progressed despite androgen-deprivation therapy.
Methods: In this double-blind, phase 3 study, we randomly assigned 1717 patients to receive either enzalutamide (at a dose of 160 mg) or placebo once daily. The coprimary end points were radiographic progression-free survival and overall survival.
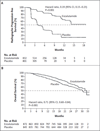
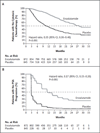
Results: The study was stopped after a planned interim analysis, conducted when 540 deaths had been reported, showed a benefit of the active treatment. The rate of radiographic progression-free survival at 12 months was 65% among patients treated with enzalutamide, as compared with 14% among patients receiving placebo (81% risk reduction; hazard ratio in the enzalutamide group, 0.19; 95% confidence interval [CI], 0.15 to 0.23; P<0.001). A total of 626 patients (72%) in the enzalutamide group, as compared with 532 patients (63%) in the placebo group, were alive at the data-cutoff date (29% reduction in the risk of death; hazard ratio, 0.71; 95% CI, 0.60 to 0.84; P<0.001). The benefit of enzalutamide was shown with respect to all secondary end points, including the time until the initiation of cytotoxic chemotherapy (hazard ratio, 0.35), the time until the first skeletal-related event (hazard ratio, 0.72), a complete or partial soft-tissue response (59% vs. 5%), the time until prostate-specific antigen (PSA) progression (hazard ratio, 0.17), and a rate of decline of at least 50% in PSA (78% vs. 3%) (P<0.001 for all comparisons). Fatigue and hypertension were the most common clinically relevant adverse events associated with enzalutamide treatment.
Conclusions: Enzalutamide significantly decreased the risk of radiographic progression and death and delayed the initiation of chemotherapy in men with metastatic prostate cancer. (Funded by Medivation and Astellas Pharma; PREVAIL ClinicalTrials.gov number, NCT01212991.).
Figures
Comment in
-
Prostate cancer: enzalutamide PREVAILs.Nat Rev Urol. 2014 Jul;11(7):361. doi: 10.1038/nrurol.2014.139. Epub 2014 Jun 17. Nat Rev Urol. 2014. PMID: 24934449 No abstract available.
-
Further analysis of PREVAIL: enzalutamide use in chemotherapy-naïve men with metastatic castration-resistant prostate cancer.Asian J Androl. 2014 Nov-Dec;16(6):803-4. doi: 10.4103/1008-682X.135129. Asian J Androl. 2014. PMID: 25080931 Free PMC article.
-
Enzalutamide in chemo-naïve castration-resistant prostate cancer: effective for most but not for all.Asian J Androl. 2014 Nov-Dec;16(6):807-8. doi: 10.4103/1008-682X.137680. Asian J Androl. 2014. PMID: 25155108 Free PMC article.
-
Word of wisdom. Re: enzalutamide in metastatic prostate cancer before chemotherapy.Eur Urol. 2014 Oct;66(4):785-6. doi: 10.1016/j.eururo.2014.07.049. Eur Urol. 2014. PMID: 25218073 No abstract available.
-
Enzalutamide in metastatic prostate cancer before chemotherapy.N Engl J Med. 2014 Oct 30;371(18):1755-6. doi: 10.1056/NEJMc1410239. N Engl J Med. 2014. PMID: 25354111 No abstract available.
-
Enzalutamide in metastatic prostate cancer before chemotherapy.N Engl J Med. 2014 Oct 30;371(18):1755. doi: 10.1056/NEJMc1410239. N Engl J Med. 2014. PMID: 25354112 No abstract available.
-
Words of wisdom. Re: Enzalutamide in metastatic prostate cancer before chemotherapy.Eur Urol. 2015 Jan;67(1):174. doi: 10.1016/j.eururo.2014.09.047. Epub 2014 Nov 25. Eur Urol. 2015. PMID: 25528395 No abstract available.
-
Enzalutamide for treatment of CRPC: rationale for sequencing and potential clinical biomarker for resistance.Cancer Biol Ther. 2015;16(2):201-3. doi: 10.4161/15384047.2014.987575. Cancer Biol Ther. 2015. PMID: 25569176 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [Erratum, CA Cancer J Clin 2011;61:134.] - PubMed
-
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. - PubMed
-
- Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. - PubMed
-
- Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. - PubMed
-
- Nair B, Wilt T, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database Syst Rev. 2002;1:CD003506. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
