Role of the primate ventral tegmental area in reinforcement and motivation
- PMID: 24881876
- PMCID: PMC4698409
- DOI: 10.1016/j.cub.2014.04.044
Role of the primate ventral tegmental area in reinforcement and motivation
Abstract
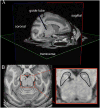
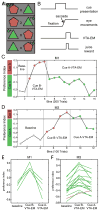
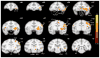
Monkey electrophysiology suggests that the activity of the ventral tegmental area (VTA) helps regulate reinforcement learning and motivated behavior, in part by broadcasting prediction error signals throughout the reward system. However, electrophysiological studies do not allow causal inferences regarding the activity of VTA neurons with respect to these processes because they require artificial manipulation of neuronal firing. Rodent studies fulfilled this requirement by demonstrating that electrical and optogenetic VTA stimulation can induce learning and modulate downstream structures. Still, the primate dopamine system has diverged significantly from that of rodents, exhibiting greatly expanded and uniquely distributed cortical and subcortical innervation patterns. Here, we bridge the gap between rodent perturbation studies and monkey electrophysiology using chronic electrical microstimulation of macaque VTA (VTA-EM). VTA-EM was found to reinforce cue selection in an operant task and to motivate future cue selection using a Pavlovian paradigm. Moreover, by combining VTA-EM with concurrent fMRI, we demonstrated that VTA-EM increased fMRI activity throughout most of the dopaminergic reward system. These results establish a causative role for primate VTA in regulating stimulus-specific reinforcement and motivation as well as in modulating activity throughout the reward system.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

