Targeting class A and C serine β-lactamases with a broad-spectrum boronic acid derivative
- PMID: 24882105
- PMCID: PMC4079326
- DOI: 10.1021/jm5006572
Targeting class A and C serine β-lactamases with a broad-spectrum boronic acid derivative
Abstract
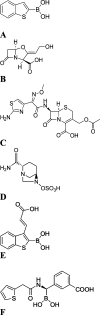
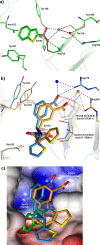
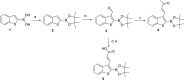
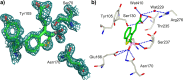
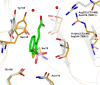
Production of β-lactamases (BLs) is the most widespread resistance mechanism adopted by bacteria to fight β-lactam antibiotics. The substrate spectrum of BLs has become increasingly broad, posing a serious health problem. Thus, there is an urgent need for novel BL inhibitors. Boronic acid transition-state analogues are able to reverse the resistance conferred by class A and C BLs. We describe a boronic acid analogue possessing interesting and potent broad-spectrum activity vs class A and C serine-based BLs. Starting from benzo(b)thiophene-2-boronic acid (BZBTH2B), a nanomolar non-β-lactam inhibitor of AmpC that can potentiate the activity of a third-generation cephalosporin against AmpC-producing resistant bacteria, we designed a novel broad-spectrum nanomolar inhibitor of class A and C BLs. Structure-based drug design (SBDD), synthesis, enzymology data, and X-ray crystallography results are discussed. We clarified the inhibitor binding geometry responsible for broad-spectrum activity vs serine-active BLs using double mutant thermodynamic cycle studies.
Figures
Similar articles
-
The complexed structure and antimicrobial activity of a non-beta-lactam inhibitor of AmpC beta-lactamase.Protein Sci. 1999 Nov;8(11):2330-7. doi: 10.1110/ps.8.11.2330. Protein Sci. 1999. PMID: 10595535 Free PMC article.
-
Computational and biological profile of boronic acids for the detection of bacterial serine- and metallo-β-lactamases.Sci Rep. 2017 Dec 18;7(1):17716. doi: 10.1038/s41598-017-17399-7. Sci Rep. 2017. PMID: 29255163 Free PMC article.
-
Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase.J Med Chem. 1998 Nov 5;41(23):4577-86. doi: 10.1021/jm980343w. J Med Chem. 1998. PMID: 9804697
-
β-Lactamase inhibitors: a review of the patent literature (2010 - 2013).Expert Opin Ther Pat. 2013 Nov;23(11):1469-81. doi: 10.1517/13543776.2013.831071. Epub 2013 Aug 23. Expert Opin Ther Pat. 2013. PMID: 23967802 Review.
-
Recent advances in the discovery of metallo-β-lactamase inhibitors for β-lactam antibiotic-resistant reversing agents.Curr Drug Targets. 2014;15(7):689-702. doi: 10.2174/1389450115666140326094504. Curr Drug Targets. 2014. PMID: 24666360 Review.
Cited by
-
First virtual screening and experimental validation of inhibitors targeting GES-5 carbapenemase.J Comput Aided Mol Des. 2019 Feb;33(2):295-305. doi: 10.1007/s10822-018-0182-2. Epub 2019 Jan 2. J Comput Aided Mol Des. 2019. PMID: 30603820
-
Structures of FOX-4 Cephamycinase in Complex with Transition-State Analog Inhibitors.Biomolecules. 2020 Apr 27;10(5):671. doi: 10.3390/biom10050671. Biomolecules. 2020. PMID: 32349291 Free PMC article.
-
Label-free fiber optic optrode for the detection of class C β-lactamases expressed by drug resistant bacteria.Biomed Opt Express. 2017 Oct 23;8(11):5191-5205. doi: 10.1364/BOE.8.005191. eCollection 2017 Nov 1. Biomed Opt Express. 2017. PMID: 29188113 Free PMC article.
-
Structural comparison of substrate-binding pockets of serine β-lactamases in classes A, C, and D.J Enzyme Inhib Med Chem. 2025 Dec;40(1):2435365. doi: 10.1080/14756366.2024.2435365. Epub 2024 Dec 23. J Enzyme Inhib Med Chem. 2025. PMID: 39714271 Free PMC article. Review.
-
Virtual screening identifies broad-spectrum β-lactamase inhibitors with activity on clinically relevant serine- and metallo-carbapenemases.Sci Rep. 2020 Jul 29;10(1):12763. doi: 10.1038/s41598-020-69431-y. Sci Rep. 2020. PMID: 32728062 Free PMC article.
References
-
- Bebrone C.; Lassaux P.; Vercheval L.; Sohier J. S.; Jehaes A.; Sauvage E.; Galleni M. Current challenges in antimicrobial chemotherapy: focus on beta-lactamase inhibition. Drugs 2010, 706651–679. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

