Effects of tumor-suppressor lysyl oxidase propeptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways
- PMID: 24882580
- PMCID: PMC4254378
- DOI: 10.1038/onc.2014.147
Effects of tumor-suppressor lysyl oxidase propeptide on prostate cancer xenograft growth and its direct interactions with DNA repair pathways
Abstract
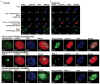
Lysyl oxidase (LOX) is a multifunctional protein required for normal collagen and elastin biosynthesis and maturation. In addition, LOX has complex roles in cancer in which the lysyl oxidase propeptide (LOX-PP) domain of secreted pro-LOX has tumor-suppressor activity, while the active enzyme promotes metastasis. In prostate cancer cell lines, recombinant LOX-PP (rLOX-PP) inhibits the growth of PC3 cells in vitro by mechanisms that were not characterized, while in DU145 cells rLOX-PP targeted fibroblast growth factor signaling. Because rLOX-PP can enhance effects of a genotoxic chemotherapeutic on breast cancer cell apoptosis, we reasoned that rLOX-PP could target DNA repair pathways typically elevated in cancer. Here we demonstrate for the first time that rLOX-PP inhibits prostate xenograft growth in vivo and that activating phosphorylations of the key DNA repair molecules ataxia-telangiectasia mutated (ATM) and checkpoint kinase 2 (CHK2) are inhibited by rLOX-PP expression in vivo. In addition, in vitro studies showed that rLOX-PP inhibits radiation-induced activating phosphorylations of ATM and CHK2 and that exogenously added rLOX-PP protein can localize to the nucleus in both DU145 and PC3 cells. rLOX-PP pull-down studies resulted in detection of a protein complex with the nuclear DNA repair regulator MRE11 in both cell lines, and rLOX-PP localized to radiation-induced nuclear DNA repair foci. Finally, rLOX-PP was shown to sensitize both DU145 and PC3 cells to radiation-induced cell death determined in colony-formation assays. These data provide evidence that rLOX-PP has a nuclear mechanism of action in which it directly interacts with DNA repair proteins to sensitize prostate cancer cells to the effects of ionizing radiation.
Conflict of interest statement
All authors declare that they have no conflicts of interest regarding the contents of this manuscript.
The authors declare that they have no conflicts of interest.
Figures
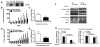



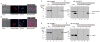

References
-
- Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. The Journal of biological chemistry. 1996;271(12):7113–9. - PubMed
-
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, et al. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. The Journal of biological chemistry. 2001;276(25):22537–43. - PubMed
-
- Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nature reviews Cancer. 2012;12(8):540–52. - PubMed
-
- Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, et al. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer research. 2007;67(3):1105–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

