Design of Functional Materials based on Liquid Crystalline Droplets
- PMID: 24882944
- PMCID: PMC4036738
- DOI: 10.1021/cm4025028
Design of Functional Materials based on Liquid Crystalline Droplets
Abstract
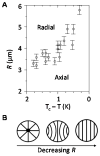
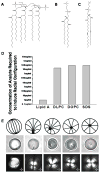
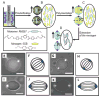
This brief perspective focuses on recent advances in the design of functional soft materials that are based on confinement of low molecular weight liquid crystals (LCs) within micrometer-sized droplets. While the ordering of LCs within micrometer-sized domains has been explored extensively in polymer-dispersed LC materials, recent studies performed with LC domains with precisely defined size and interfacial chemistry have unmasked observations of confinement-induced ordering of LCs that do not follow previously reported theoretical predictions. These new findings, which are enabled in part by advances in the preparation of LCs encapsulated in polymeric shells, are opening up new opportunities for the design of soft responsive materials based on surface-induced ordering transitions. These materials are also providing new insights into the self-assembly of biomolecular and colloidal species at defects formed by LCs confined to micrometer-sized domains. The studies presented in this perspective serve additionally to highlight gaps in knowledge regarding the ordering of LCs in confined systems.
Keywords: Liquid crystals; amphiphiles; chemically patterned microparticles; functional materials; micrometer-sized droplets; ordering transitions; self-assembly; topological defects.
Figures







Similar articles
-
Recent advances in colloidal and interfacial phenomena involving liquid crystals.Langmuir. 2011 May 17;27(10):5719-38. doi: 10.1021/la103301d. Epub 2010 Nov 19. Langmuir. 2011. PMID: 21090596 Free PMC article. Review.
-
Characterization of adsorbate-induced ordering transitions of liquid crystals within monodisperse droplets.Langmuir. 2009 Aug 18;25(16):9016-24. doi: 10.1021/la900786b. Langmuir. 2009. PMID: 19719217
-
Dynamic Microparticle Assembly at the Interface of Chemoresponsive Liquid Crystal Droplets.Anal Chem. 2024 Mar 5;96(9):3780-3786. doi: 10.1021/acs.analchem.3c04555. Epub 2024 Feb 26. Anal Chem. 2024. PMID: 38407028
-
Chemically responsive gels prepared from microspheres dispersed in liquid crystals.Small. 2009 Nov;5(22):2589-96. doi: 10.1002/smll.200900961. Small. 2009. PMID: 19777464
-
Design of Responsive and Active (Soft) Materials Using Liquid Crystals.Annu Rev Chem Biomol Eng. 2016 Jun 7;7:163-96. doi: 10.1146/annurev-chembioeng-061114-123323. Epub 2016 Mar 10. Annu Rev Chem Biomol Eng. 2016. PMID: 26979412 Free PMC article. Review.
Cited by
-
A New Strategy for Reporting Specific Protein Binding Events at Aqueous-Liquid Crystal Interfaces in the Presence of Non-Specific Proteins.ACS Appl Mater Interfaces. 2020 Feb 19;12(7):7869-7878. doi: 10.1021/acsami.9b16867. Epub 2020 Feb 7. ACS Appl Mater Interfaces. 2020. PMID: 31825195 Free PMC article.
-
Ultra-stable liquid crystal droplets coated by sustainable plant-based materials for optical sensing of chemical and biological analytes.J Mater Chem C Mater. 2023 Apr 11;11(17):5831-5845. doi: 10.1039/d3tc00598d. eCollection 2023 May 4. J Mater Chem C Mater. 2023. PMID: 37153011 Free PMC article.
-
Tunable Gas Sensing Gels by Cooperative Assembly.Adv Funct Mater. 2017 Jul 19;27(27):1700803. doi: 10.1002/adfm.201700803. Adv Funct Mater. 2017. PMID: 28747856 Free PMC article.
-
Chiral nematic liquid crystal droplets as a basis for sensor systems.Mol Syst Des Eng. 2022 Mar 7;7(6):607-621. doi: 10.1039/d1me00189b. eCollection 2022 Jun 6. Mol Syst Des Eng. 2022. PMID: 36876150 Free PMC article.
-
Multiresponsive polymeric microstructures with encoded predetermined and self-regulated deformability.Proc Natl Acad Sci U S A. 2018 Dec 18;115(51):12950-12955. doi: 10.1073/pnas.1811823115. Epub 2018 Dec 4. Proc Natl Acad Sci U S A. 2018. PMID: 30514819 Free PMC article.
References
-
- Chandrasekhar S. Liquid crystals. Cambridge University Press; Cambridge [England]; New York, NY, USA: 1992.
-
- Collings PJ. Liquid crystals : nature’s delicate phase of matter. Princeton University Press; Princeton, N.J: 2002.
-
- Collings PJ, Hird M. Introduction to liquid crystals chemistry and physics. Taylor & Francis; London; Bristol, PA: 1997.
-
- Gennes P-Gd, Prost J. The physics of liquid crystals. Clarendon Press; Oxford University Press; Oxford New York: 1993.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
