Korean guidelines for the diagnosis and management of dry eye: development and validation of clinical efficacy
- PMID: 24882952
- PMCID: PMC4038724
- DOI: 10.3341/kjo.2014.28.3.197
Korean guidelines for the diagnosis and management of dry eye: development and validation of clinical efficacy
Abstract
Purpose: To evaluate the clinical efficacy of newly developed guidelines for the diagnosis and management of dry eye.
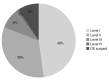
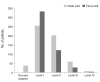
Methods: This retrospective, multi-center, non-randomized, observational study included a total of 1,612 patients with dry eye disease who initially visited the clinics from March 2010 to August 2010. Korean guidelines for the diagnosis and management of dry eye were newly developed from concise, expert-consensus recommendations. Severity levels at initial and final visits were determined using the guidelines in patients with 90 ± 7 days of follow-up visits (n = 526). Groups with different clinical outcomes were compared with respect to clinical parameters, treatment modalities, and guideline compliance. Main outcome measures were ocular and visual symptoms, ocular surface disease index, global assessment by patient and physician, tear film break-up time, Schirmer-1 test score, ocular surface staining score at initial and final visits, clinical outcome after three months of treatment, and guideline compliance.
Results: Severity level was reduced in 47.37% of patients treated as recommended by the guidelines. Younger age (odd ratio [OR], 0.984; p = 0.044), higher severity level at initial visit, compliance to treatment recommendation (OR, 1.832; p = 0.047), and use of topical cyclosporine (OR, 1.838; p = 0.011) were significantly associated with improved clinical outcomes.
Conclusions: Korean guidelines for the diagnosis and management of dry eye can be used as a valid and effective tool for the treatment of dry eye disease.
Keywords: Dry eye syndrome; Practice guideline; Standards.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232. - PubMed
-
- Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–907. - PubMed
-
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. - PubMed
-
- Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:108–152. - PubMed
-
- Han SB, Hyon JY, Woo SJ, et al. Prevalence of dry eye disease in an elderly Korean population. Arch Ophthalmol. 2011;129:633–638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

