The usefulness of interferon-gamma release assay for diagnosis of tuberculosis-related uveitis in Korea
- PMID: 24882956
- PMCID: PMC4038728
- DOI: 10.3341/kjo.2014.28.3.226
The usefulness of interferon-gamma release assay for diagnosis of tuberculosis-related uveitis in Korea
Abstract
Purpose: To evaluate the usefulness of the interferon-gamma release assay (IGRA) for diagnosing tuberculosis (TB)-related uveitis (TRU).
Methods: Records from 181 patients with ocular signs and symptoms suggestive of TRU and intraocular inflammation of unknown etiology were reviewed. All subjects underwent clinical and laboratory testing, including IGRA, to rule out presence of underlying disease. A diagnosis of presumed TRU was made based on an internist's TB diagnosis and a patient's response to anti-TB therapy. Sensitivity, specificity, and positive predictive values of IGRA for TRU diagnosis were calculated. Clinical characteristics were compared between patients with positive and negative IGRA results.
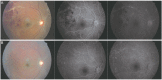
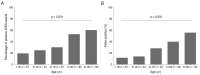
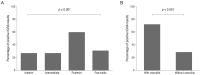
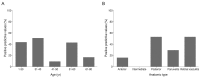
Results: The sensitivity and specificity of IGRA for TRU were 100% and 72.0%, respectively. Mean age, percentage of patients with retinal vasculitis, and the anatomic type of uveitis were significantly different between patients with positive and negative IGRA results (all p ≤ 0.001). Positive IGRA rates and false-positive rates were significantly different between age and anatomic type groups (both p = 0.001). The positive predictive value of the IGRA among patients with intraocular inflammation was high (70%) when all of younger age (≤ 40 years), posterior uveitis, and retinal vasculitis were present.
Conclusions: The IGRA is useful for diagnosing TRU in the Korean population, especially when it is used as a screening test. Clinical characteristics, including younger age (≤ 40 years), posterior uveitis, and retinal vasculitis in IGRA-positive patients, increase the likelihood of the patient having TRU.
Keywords: Age; Interferon-gamma release tests; Retinal vasculitis; Tuberculosis; Uveitis.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Indirect supportive evidence for diagnosis of tuberculosis-related uveitis: from the tuberculin skin test to the new interferon gamma release assays.Acta Ophthalmol. 2013 Mar;91(2):e99-e107. doi: 10.1111/j.1755-3768.2012.02564.x. Epub 2012 Oct 8. Acta Ophthalmol. 2013. PMID: 23039176
-
Interferon-gamma release assay as a diagnostic test for tuberculosis-associated uveitis.Eye (Lond). 2012 May;26(5):658-65. doi: 10.1038/eye.2012.1. Epub 2012 Feb 3. Eye (Lond). 2012. PMID: 22302066 Free PMC article.
-
Discordance of two interferon-γ release assays and tuberculin skin test in patients with uveitis.Br J Ophthalmol. 2014 Dec;98(12):1649-53. doi: 10.1136/bjophthalmol-2014-305229. Epub 2014 Jul 7. Br J Ophthalmol. 2014. PMID: 25001322
-
Update on Immunological Test (Quantiferon-TB Gold) Contribution in the Management of Tuberculosis-Related Ocular Inflammation.Ocul Immunol Inflamm. 2018;26(8):1192-1199. doi: 10.1080/09273948.2017.1332232. Epub 2017 Jul 12. Ocul Immunol Inflamm. 2018. PMID: 28700283 Review.
-
Uveitis with occult choroiditis due to Mycobacterium kansasii: limitations of interferon-gamma release assay (IGRA) tests (case report and mini-review on ocular non-tuberculous mycobacteria and IGRA cross-reactivity).Int Ophthalmol. 2012 Oct;32(5):499-506. doi: 10.1007/s10792-012-9588-3. Epub 2012 Jun 2. Int Ophthalmol. 2012. PMID: 22661050 Review.
Cited by
-
Contribution of Dual Fluorescein and Indocyanine Green Angiography to the Appraisal of Presumed Tuberculous Chorioretinitis in a Non-endemic Area.J Ophthalmic Vis Res. 2017 Jan-Mar;12(1):30-38. doi: 10.4103/2008-322X.200157. J Ophthalmic Vis Res. 2017. PMID: 28299004 Free PMC article.
-
The Association between QuantiFERON-TB Gold Test and Clinical Manifestations of Uveitis in the United States.Am J Ophthalmol. 2021 Oct;230:181-187. doi: 10.1016/j.ajo.2021.04.024. Epub 2021 May 2. Am J Ophthalmol. 2021. PMID: 33945821 Free PMC article.
-
State-of-the-Art Review: Ocular Infections.Clin Infect Dis. 2024 Nov 22;79(5):e48-e64. doi: 10.1093/cid/ciae433. Clin Infect Dis. 2024. PMID: 39571607
-
Diagnosis and biomarkers for ocular tuberculosis: From the present into the future.Theranostics. 2023 Apr 1;13(7):2088-2113. doi: 10.7150/thno.81488. eCollection 2023. Theranostics. 2023. PMID: 37153734 Free PMC article. Review.
-
What does IGRA testing add to the diagnosis of ocular tuberculosis? A Bayesian latent class analysis.BMC Ophthalmol. 2017 Dec 8;17(1):245. doi: 10.1186/s12886-017-0597-x. BMC Ophthalmol. 2017. PMID: 29216851 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC) Trends in tuberculosis: United States, 2004. MMWR Morb Mortal Wkly Rep. 2005;54:245–249. - PubMed
-
- Dye C, Scheele S, Dolin P, et al. WHO Global Surveillance and Monitoring Project. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Expanded tuberculosis surveillance and tuberculosis morbidity: United States, 1993. MMWR Morb Mortal Wkly Rep. 1994;43:361–366. - PubMed
-
- Tabbara KF. Tuberculosis. Curr Opin Ophthalmol. 2007;18:493–501. - PubMed
-
- Kang YA, Lee HW, Yoon HI, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–2761. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

