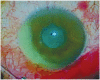
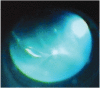
Suspected bacterial endophthalmitis following sustained-release dexamethasone intravitreal implant: a case report
- PMID: 24882964
- PMCID: PMC4038736
- DOI: 10.3341/kjo.2014.28.3.275
Suspected bacterial endophthalmitis following sustained-release dexamethasone intravitreal implant: a case report
Abstract
A 58-year-old man admitted to our opthalmology department with the complaint of branch retinal vein occlusion. He was treated with intravitreal Ozurdex in the right eye. Two days after the injection, the patient presented with ocular pain and the visual acuity was hand movement. A diagnosis of endophthalmitis was made. We performed emergent pars plana vitrectomy (PPV) and the implant was removed from the vitreous cavity using a retinal forceps. A combination of vancomycin 1.0 mg and amikacin 0.4 mg was injected intravitreally. However, because of the blurring in the vitreus one week after the procedure, phacoemulsification and a repeat PPV was performed. Five days after the last procedure the signs and symptoms of endophthalmitis were resolved. Our case demonstrated that endophthalmitis could develop after intravitreal implantation of Ozurdex. Surgical removal of the implant and immediate vitrectomy seems to be a useful treatment option in these cases.
Keywords: Dexamethasone implant; Endophthalmitis; Ozurdex; Pars plana vitrectomy.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Drug-resistant coagulase-negative staphylococcal endophthalmitis following dexamethasone intravitreal implant.Indian J Ophthalmol. 2017 Jul;65(7):634-636. doi: 10.4103/ijo.IJO_810_16. Indian J Ophthalmol. 2017. PMID: 28724830 Free PMC article.
-
Staphylococcus lugdunensis endophthalmitis following dexamethasone intravitreal implant.Indian J Ophthalmol. 2019 Mar;67(3):424-426. doi: 10.4103/ijo.IJO_720_18. Indian J Ophthalmol. 2019. PMID: 30777977 Free PMC article.
-
Atypical Delayed-Onset Endophthalmitis Following Intravitreal Dexamethasone Implant Managed Without Implant Removal: A Rare Case Report and Literature Review.Rom J Ophthalmol. 2024 Oct-Dec;68(4):343-348. doi: 10.22336/rjo.2024.64. Rom J Ophthalmol. 2024. PMID: 39936053 Free PMC article. Review.
-
TWO CASES OF ACUTE ENDOPHTHALMITIS AFTER INTRAVITREAL DEXAMETHASONE IMPLANT INJECTION.Retin Cases Brief Rep. 2016 Spring;10(2):154-6. doi: 10.1097/ICB.0000000000000213. Retin Cases Brief Rep. 2016. PMID: 26426482
-
CASE REPORT OF ABIOTROPHIA DEFECTIVA ENDOPHTHALMITIS AFTER REPEATED INJECTIONS OF DEXAMETHASONE INTRAVITREAL IMPLANT (OZURDEX).Retin Cases Brief Rep. 2022 Mar 1;16(2):170-173. doi: 10.1097/ICB.0000000000000925. Retin Cases Brief Rep. 2022. PMID: 31479011 Review.
Cited by
-
Commentary: Endophthalmitis following intravitreal injection of dexamethasone implant.Indian J Ophthalmol. 2019 Mar;67(3):426-428. doi: 10.4103/ijo.IJO_1809_18. Indian J Ophthalmol. 2019. PMID: 30777978 Free PMC article. No abstract available.
-
Acute bacterial endophthalmitis following intravitreal dexamethasone implant: A case report and review of literature.Saudi J Ophthalmol. 2017 Jan-Mar;31(1):51-54. doi: 10.1016/j.sjopt.2016.12.003. Epub 2016 Dec 18. Saudi J Ophthalmol. 2017. PMID: 28337065 Free PMC article.
-
Drug-resistant coagulase-negative staphylococcal endophthalmitis following dexamethasone intravitreal implant.Indian J Ophthalmol. 2017 Jul;65(7):634-636. doi: 10.4103/ijo.IJO_810_16. Indian J Ophthalmol. 2017. PMID: 28724830 Free PMC article.
-
Staphylococcus lugdunensis endophthalmitis following dexamethasone intravitreal implant.Indian J Ophthalmol. 2019 Mar;67(3):424-426. doi: 10.4103/ijo.IJO_720_18. Indian J Ophthalmol. 2019. PMID: 30777977 Free PMC article.
-
Incomplete scleral penetration of dexamethasone (Ozurdex) intravitreal implant.BMJ Case Rep. 2018 Dec 13;11(1):e227055. doi: 10.1136/bcr-2018-227055. BMJ Case Rep. 2018. PMID: 30567246 Free PMC article.
References
-
- Schmitz K, Maier M, Clemens CR, et al. The ZERO study. Reliability and safety of intravitreal Ozurdex injections. Ophthalmologe. 2014;111:44–52. - PubMed
-
- Haller JA, Bandello F, Belfort R, Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. - PubMed
-
- London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011;28:351–366. - PubMed
-
- Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous