Protein redox modification as a cellular defense mechanism against tissue ischemic injury
- PMID: 24883175
- PMCID: PMC4026984
- DOI: 10.1155/2014/343154
Protein redox modification as a cellular defense mechanism against tissue ischemic injury
Abstract
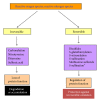
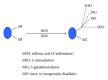
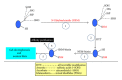
Protein oxidative or redox modifications induced by reactive oxygen species (ROS) or reactive nitrogen species (RNS) not only can impair protein function, but also can regulate and expand protein function under a variety of stressful conditions. Protein oxidative modifications can generally be classified into two categories: irreversible oxidation and reversible oxidation. While irreversible oxidation usually leads to protein aggregation and degradation, reversible oxidation that usually occurs on protein cysteine residues can often serve as an "on and off" switch that regulates protein function and redox signaling pathways upon stress challenges. In the context of ischemic tolerance, including preconditioning and postconditioning, increasing evidence has indicated that reversible cysteine redox modifications such as S-sulfonation, S-nitrosylation, S-glutathionylation, and disulfide bond formation can serve as a cellular defense mechanism against tissue ischemic injury. In this review, I highlight evidence of cysteine redox modifications as protective measures in ischemic injury, demonstrating that protein redox modifications can serve as a therapeutic target for attenuating tissue ischemic injury. Prospectively, more oxidatively modified proteins will need to be identified that can play protective roles in tissue ischemic injury, in particular, when the oxidative modifications of such identified proteins can be enhanced by pharmacological agents or drugs that are available or to be developed.
Figures
Similar articles
-
Redox proteomics: from bench to bedside.Adv Exp Med Biol. 2014;806:301-17. doi: 10.1007/978-3-319-06068-2_13. Adv Exp Med Biol. 2014. PMID: 24952188 Review.
-
Pathological Impact of Redox Post-Translational Modifications.Antioxid Redox Signal. 2024 Jul;41(1-3):152-180. doi: 10.1089/ars.2023.0252. Epub 2024 Apr 8. Antioxid Redox Signal. 2024. PMID: 38504589 Review.
-
A direct way of redox sensing.RNA Biol. 2011 Jan-Feb;8(1):18-23. doi: 10.4161/rna.8.1.13555. Epub 2011 Jan 1. RNA Biol. 2011. PMID: 21220941
-
ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications.Free Radic Res. 2018 May;52(5):507-543. doi: 10.1080/10715762.2018.1457217. Epub 2018 Apr 19. Free Radic Res. 2018. PMID: 29589770 Review.
-
Introduction to the thematic minireview series on redox-active protein modifications and signaling.J Biol Chem. 2013 Sep 13;288(37):26463. doi: 10.1074/jbc.R113.502583. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861402 Free PMC article.
Cited by
-
Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer.Mutat Res Rev Mutat Res. 2021 Jan-Jun;787:108365. doi: 10.1016/j.mrrev.2021.108365. Epub 2021 Jan 11. Mutat Res Rev Mutat Res. 2021. PMID: 34083039 Free PMC article. Review.
-
Thiol/disulfide homeostasis as a novel indicator of oxidative stress during the treatment process of patients with septic arthritis.Jt Dis Relat Surg. 2020;31(3):502-508. doi: 10.5606/ehc.2020.71982. Jt Dis Relat Surg. 2020. PMID: 32962582 Free PMC article.
-
Potential Biochemical Mechanisms of Lung Injury in Diabetes.Aging Dis. 2017 Feb 1;8(1):7-16. doi: 10.14336/AD.2016.0627. eCollection 2017 Feb. Aging Dis. 2017. PMID: 28203478 Free PMC article. Review.
-
Total flavonoids of Selaginella tamariscina (P. Beauv.) Spring ameliorates diabetes-induced acute lung injury via activating Nrf2/HO-1.Iran J Basic Med Sci. 2024;27(11):1423-1429. doi: 10.22038/ijbms.2024.79246.17166. Iran J Basic Med Sci. 2024. PMID: 39386236 Free PMC article.
-
Mitochondrial Dihydrolipoamide Dehydrogenase is Upregulated in Response to Intermittent Hypoxic Preconditioning.Int J Med Sci. 2015 May 23;12(5):432-40. doi: 10.7150/ijms.11402. eCollection 2015. Int J Med Sci. 2015. PMID: 26078703 Free PMC article.
References
-
- Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxidants and Redox Signaling. 2009;11(11):2655–2671. - PubMed
-
- Groeger G, Doonan F, Cotter TG, Donovan M. Reactive oxygen species regulate prosurvival ERK1/2 signaling and bFGF expression in gliosis within the retina. Investigative Ophthalmology & Visual Science. 2012;53:6645–6654. - PubMed
-
- Bevilacqua E, Gomes SZ, Lorenzon AR, Hoshida MS, Amarante-Paffaro AM. NADPH oxidase as an important source of reactive oxygen species at the mouse maternal-fetal interface: putative biological roles. Reproductive BioMedicine Online. 2012;25:31–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

