Nanotechnology biomimetic cartilage regenerative scaffolds
- PMID: 24883273
- PMCID: PMC4037768
- DOI: 10.5999/aps.2014.41.3.231
Nanotechnology biomimetic cartilage regenerative scaffolds
Abstract
Cartilage has a limited regenerative capacity. Faced with the clinical challenge of reconstruction of cartilage defects, the field of cartilage engineering has evolved. This article reviews current concepts and strategies in cartilage engineering with an emphasis on the application of nanotechnology in the production of biomimetic cartilage regenerative scaffolds. The structural architecture and composition of the cartilage extracellular matrix and the evolution of tissue engineering concepts and scaffold technology over the last two decades are outlined. Current advances in biomimetic techniques to produce nanoscaled fibrous scaffolds, together with innovative methods to improve scaffold biofunctionality with bioactive cues are highlighted. To date, the majority of research into cartilage regeneration has been focused on articular cartilage due to the high prevalence of large joint osteoarthritis in an increasingly aging population. Nevertheless, the principles and advances are applicable to cartilage engineering for plastic and reconstructive surgery.
Keywords: Biomimetics; Cartilage; Guided tissue regeneration; Nanotechnology; Tissue scaffolds.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



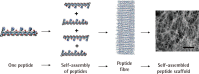

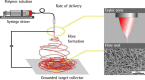

References
-
- Wheater PR, Burkitt HG. Skeletal tissues. In: Wheater PR, Burkitt HG, Daniels VG, editors. Functional histology. 2nd ed. Edinburgh: Churchill Livingston; 1987. pp. 142–160.
-
- Lane AP. Nasal anatomy and physiology. Facial Plast Surg Clin North Am. 2004;12:387–395. - PubMed
-
- Ross MH, Kaye GI, Pawlina W. Cartilage. In: Ross MH, Pawlina W, editors. Histology: a text and atlas. Baltimore: Lippincott Williams & Wilkins; 2002. pp. 164–179.
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

