Osteoconductive potential of barrier nanoSiO2 PLGA membranes functionalized by plasma enhanced chemical vapour deposition
- PMID: 24883304
- PMCID: PMC4026916
- DOI: 10.1155/2014/253590
Osteoconductive potential of barrier nanoSiO2 PLGA membranes functionalized by plasma enhanced chemical vapour deposition
Abstract
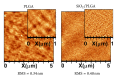
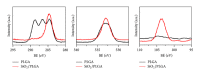
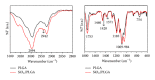
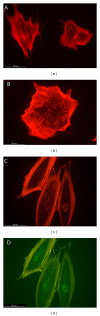
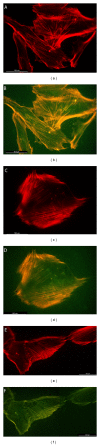
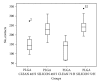
The possibility of tailoring membrane surfaces with osteoconductive potential, in particular in biodegradable devices, to create modified biomaterials that stimulate osteoblast response should make them more suitable for clinical use, hopefully enhancing bone regeneration. Bioactive inorganic materials, such as silica, have been suggested to improve the bioactivity of synthetic biopolymers. An in vitro study on HOB human osteoblasts was performed to assess biocompatibility and bioactivity of SiO2 functionalized poly(lactide-co-glycolide) (PLGA) membranes, prior to clinical use. A 15 nm SiO2 layer was deposited by plasma enhanced chemical vapour deposition (PECVD), onto a resorbable PLGA membrane. Samples were characterized by X-ray photoelectron spectroscopy, atomic force microscopy, scanning electron microscopy, and infrared spectroscopy (FT-IR). HOB cells were seeded on sterilized test surfaces where cell morphology, spreading, actin cytoskeletal organization, and focal adhesion expression were assessed. As proved by the FT-IR analysis of samples, the deposition by PECVD of the SiO2 onto the PLGA membrane did not alter the composition and other characteristics of the organic membrane. A temporal and spatial reorganization of cytoskeleton and focal adhesions and morphological changes in response to SiO2 nanolayer were identified in our model. The novedous SiO2 deposition method is compatible with the standard sterilization protocols and reveals as a valuable tool to increase bioactivity of resorbable PLGA membranes.
Figures


Similar articles
-
Osteoblasts Interaction with PLGA Membranes Functionalized with Titanium Film Nanolayer by PECVD. In vitro Assessment of Surface Influence on Cell Adhesion during Initial Cell to Material Interaction.Materials (Basel). 2014 Mar 4;7(3):1687-1708. doi: 10.3390/ma7031687. Materials (Basel). 2014. PMID: 28788538 Free PMC article.
-
Nanoroughness, Surface Chemistry, and Drug Delivery Control by Atmospheric Plasma Jet on Implantable Devices.ACS Appl Mater Interfaces. 2018 Nov 21;10(46):39512-39523. doi: 10.1021/acsami.8b15886. Epub 2018 Nov 12. ACS Appl Mater Interfaces. 2018. PMID: 30359523
-
Comparative evaluation of the biocompatible and physical-chemical properties of poly(lactide-co-glycolide) and polydopamine as coating materials for bacterial cellulose.J Mater Chem B. 2019 Jan 28;7(4):630-639. doi: 10.1039/c8tb02456a. Epub 2019 Jan 4. J Mater Chem B. 2019. PMID: 32254796
-
In vivo comparative model of oxygen plasma and nanocomposite particles on PLGA membranes for guided bone regeneration processes to be applied in pre-prosthetic surgery: a pilot study.J Dent. 2014 Nov;42(11):1446-57. doi: 10.1016/j.jdent.2014.04.015. J Dent. 2014. PMID: 24814137
-
Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: in vitro and in vivo characteristics.J Biomed Mater Res A. 2015 Nov;103(11):3580-9. doi: 10.1002/jbm.a.35499. Epub 2015 May 29. J Biomed Mater Res A. 2015. PMID: 25969423
Cited by
-
Trends in bioactivity: inducing and detecting mineralization of regenerative polymeric scaffolds.J Mater Chem B. 2024 Mar 13;12(11):2720-2736. doi: 10.1039/d3tb02674d. J Mater Chem B. 2024. PMID: 38410921 Free PMC article. Review.
-
In Vitro and in Vivo Study of Poly(Lactic⁻co⁻Glycolic) (PLGA) Membranes Treated with Oxygen Plasma and Coated with Nanostructured Hydroxyapatite Ultrathin Films for Guided Bone Regeneration Processes.Polymers (Basel). 2017 Sep 2;9(9):410. doi: 10.3390/polym9090410. Polymers (Basel). 2017. PMID: 30965714 Free PMC article.
-
Comparative evaluation of coronally advanced flap with and without Biomesh® membrane for the treatment of localized gingival recession defects - a clinical study.J Med Life. 2022 May;15(5):705-716. doi: 10.25122/jml-2021-0109. J Med Life. 2022. PMID: 35815079 Free PMC article.
-
Controlled-release of tetracycline and lovastatin by poly(D,L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs.Int J Nanomedicine. 2016 Jan 18;11:285-97. doi: 10.2147/IJN.S94270. eCollection 2016. Int J Nanomedicine. 2016. PMID: 26848264 Free PMC article.
-
In Vitro Comparative Study of Oxygen Plasma Treated Poly(Lactic⁻Co⁻Glycolic) (PLGA) Membranes and Supported Nanostructured Oxides for Guided Bone Regeneration Processes.Materials (Basel). 2018 May 8;11(5):752. doi: 10.3390/ma11050752. Materials (Basel). 2018. PMID: 29738457 Free PMC article.
References
-
- Lee E-J, Teng S-H, Jang T-S, et al. Nanostructured poly(ε-caprolactone)-silica xerogel fibrous membrane for guided bone regeneration. Acta Biomaterialia. 2010;6(9):3557–3565. - PubMed
-
- Orita T, Tomita M, Kato K. Regulation of cellular responses to macroporous inorganic films prepared by the inverse-opal method. Colloids and Surfaces B: Biointerfaces. 2011;84(1):187–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

