Fatty acids in energy metabolism of the central nervous system
- PMID: 24883315
- PMCID: PMC4026875
- DOI: 10.1155/2014/472459
Fatty acids in energy metabolism of the central nervous system
Abstract
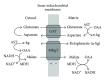
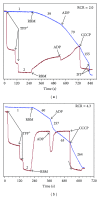
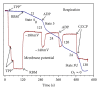
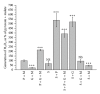
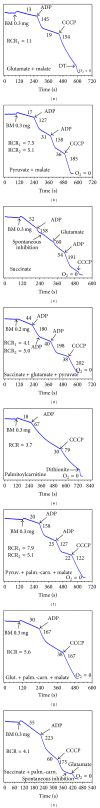
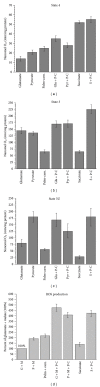
In this review, we analyze the current hypotheses regarding energy metabolism in the neurons and astroglia. Recently, it was shown that up to 20% of the total brain's energy is provided by mitochondrial oxidation of fatty acids. However, the existing hypotheses consider glucose, or its derivative lactate, as the only main energy substrate for the brain. Astroglia metabolically supports the neurons by providing lactate as a substrate for neuronal mitochondria. In addition, a significant amount of neuromediators, glutamate and GABA, is transported into neurons and also serves as substrates for mitochondria. Thus, neuronal mitochondria may simultaneously oxidize several substrates. Astrocytes have to replenish the pool of neuromediators by synthesis de novo, which requires large amounts of energy. In this review, we made an attempt to reconcile β-oxidation of fatty acids by astrocytic mitochondria with the existing hypothesis on regulation of aerobic glycolysis. We suggest that, under condition of neuronal excitation, both metabolic pathways may exist simultaneously. We provide experimental evidence that isolated neuronal mitochondria may oxidize palmitoyl carnitine in the presence of other mitochondrial substrates. We also suggest that variations in the brain mitochondrial metabolic phenotype may be associated with different mtDNA haplogroups.
Figures

Similar articles
-
Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems.FASEB J. 2016 May;30(5):1913-26. doi: 10.1096/fj.201500182. Epub 2016 Feb 2. FASEB J. 2016. PMID: 26839375
-
GSEA of mouse and human mitochondriomes reveals fatty acid oxidation in astrocytes.Glia. 2018 Aug;66(8):1724-1735. doi: 10.1002/glia.23330. Epub 2018 Mar 25. Glia. 2018. PMID: 29575211
-
Mitochondrial tissue specificity of substrates utilization in rat cardiac and skeletal muscles.J Cell Physiol. 2005 Jun;203(3):479-86. doi: 10.1002/jcp.20245. J Cell Physiol. 2005. PMID: 15521069
-
Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte-neuron lactate shuttle in brain.J Neurosci Res. 2017 Nov;95(11):2103-2125. doi: 10.1002/jnr.24015. Epub 2017 Feb 2. J Neurosci Res. 2017. PMID: 28151548 Review.
-
Glucose and lactate metabolism during brain activation.J Neurosci Res. 2001 Dec 1;66(5):824-38. doi: 10.1002/jnr.10079. J Neurosci Res. 2001. PMID: 11746408 Review.
Cited by
-
Excessive lipid production shapes glioma tumor microenvironment.Ultrastruct Pathol. 2024 Sep 2;48(5):367-377. doi: 10.1080/01913123.2024.2392728. Epub 2024 Aug 19. Ultrastruct Pathol. 2024. PMID: 39157967
-
Long-Chain and Medium-Chain Fatty Acids in Energy Metabolism of Murine Kidney Mitochondria.Int J Mol Sci. 2022 Dec 26;24(1):379. doi: 10.3390/ijms24010379. Int J Mol Sci. 2022. PMID: 36613826 Free PMC article.
-
A New Hypothesis for Alzheimer's Disease: The Lipid Invasion Model.J Alzheimers Dis Rep. 2022 Mar 25;6(1):129-161. doi: 10.3233/ADR-210299. eCollection 2022. J Alzheimers Dis Rep. 2022. PMID: 35530118 Free PMC article.
-
Convergent Canonical Pathways in Autism Spectrum Disorder from Proteomic, Transcriptomic and DNA Methylation Data.Int J Mol Sci. 2021 Oct 5;22(19):10757. doi: 10.3390/ijms221910757. Int J Mol Sci. 2021. PMID: 34639097 Free PMC article. Review.
-
The Physiological and Pathological Role of Acyl-CoA Oxidation.Int J Mol Sci. 2023 Oct 3;24(19):14857. doi: 10.3390/ijms241914857. Int J Mol Sci. 2023. PMID: 37834305 Free PMC article. Review.
References
-
- Jenuth JP, Peterson AC, Fu K, Shoubridge EA. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nature Genetics. 1996;14(2):146–151. - PubMed
-
- Wallace DC. Why do we still have a maternally inherited mitochondrial DNA? insights from evolutionary medicine. Annual Review of Biochemistry. 2007;76:781–821. - PubMed
-
- Darvishi K, Sharma S, Bhat AK, Rai E, Bamezai RNK. Mitochondrial DNA G10398A polymorphism imparts maternal Haplogroup N a risk for breast and esophageal cancer. Cancer Letters. 2007;249(2):249–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

