Synthesis and photophysical and electrochemical properties of functionalized mono-, bis-, and trisanthracenyl bridged Ru(II) bis(2,2':6',2"-terpyridine) charge transfer complexes
- PMID: 24883408
- PMCID: PMC4032701
- DOI: 10.1155/2014/570864
Synthesis and photophysical and electrochemical properties of functionalized mono-, bis-, and trisanthracenyl bridged Ru(II) bis(2,2':6',2"-terpyridine) charge transfer complexes
Abstract
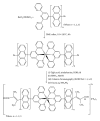
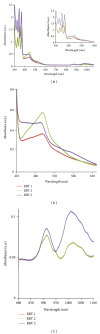
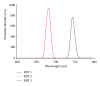
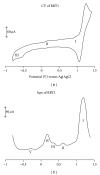
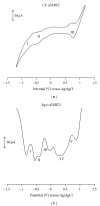
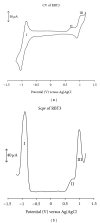
With the aim of developing new molecular devices having long-range electron transfer in artificial systems and as photosensitizers, a series of homoleptic ruthenium(II) bisterpyridine complexes bearing one to three anthracenyl units sandwiched between terpyridine and 2-methyl-2-butenoic acid group are synthesized and characterized. The complexes formulated as bis-4'-(9-monoanthracenyl-10-(2-methyl-2-butenoic acid) terpyridyl) ruthenium(II) bis(hexafluorophosphate) (RBT1), bis-4'-(9-dianthracenyl-10-(2-methyl-2-butenoic acid) terpyridyl) ruthenium(II) bis(hexafluorophosphate) (RBT2), and bis-4'-(9-trianthracenyl-10-(2-methyl-2-butenoic acid) terpyridyl) ruthenium(II) bis(hexafluorophosphate) (RBT3) were characterized by elemental analysis, FT-IR, UV-Vis, photoluminescence, (1)H and (13)C NMR spectroscopy, and electrochemical techniques by elemental analysis, FT-IR, UV-Vis, photoluminescence, (1)H and (13)C NMR spectroscopy, and electrochemical techniques. The cyclic voltammograms (CVs) of (RBT1), (RBT2), and (RBT3) display reversible one-electron oxidation processes at E 1/2 = 1.13 V, 0.71 V, and 0.99 V, respectively (versus Ag/AgCl). Based on a general linear correlation between increase in the length of π-conjugation bond and the molar extinction coefficients, the Ru(II) bisterpyridyl complexes show characteristic broad and intense metal-to-ligand charge transfer (MLCT) band absorption transitions between 480-600 nm, ε = 9.45 × 10(3) M(-1) cm(-1), and appreciable photoluminescence spanning the visible region.
Figures
Similar articles
-
A high molar extinction coefficient bisterpyridyl homoleptic ru(II) complex with trans-2-methyl-2-butenoic acid functionality: potential dye for dye-sensitized solar cells.Int J Mol Sci. 2012;13(3):3511-3526. doi: 10.3390/ijms13033511. Epub 2012 Mar 14. Int J Mol Sci. 2012. PMID: 22489165 Free PMC article.
-
A high molar extinction coefficient mono-anthracenyl bipyridyl heteroleptic ruthenium(II) complex: synthesis, photophysical and electrochemical properties.Molecules. 2011 Jun 3;16(6):4615-31. doi: 10.3390/molecules16064615. Molecules. 2011. PMID: 21642936 Free PMC article.
-
Synthesis and characterization of a Ru(II) complex with functionalized phenanthroline ligands having single-double linked anthracenyl and 1-methoxy-1-buten-3-yne moieties.Molecules. 2010 Oct 27;15(11):7570-81. doi: 10.3390/molecules15117570. Molecules. 2010. PMID: 21030910 Free PMC article.
-
Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy.Acc Chem Res. 2017 Nov 21;50(11):2727-2736. doi: 10.1021/acs.accounts.7b00180. Epub 2017 Oct 23. Acc Chem Res. 2017. PMID: 29058879 Review.
-
Effect of Remote Amine Groups on Ground- and Excited-State Properties of Terpyridyl d-Metal Complexes.Molecules. 2025 May 29;30(11):2386. doi: 10.3390/molecules30112386. Molecules. 2025. PMID: 40509271 Free PMC article. Review.
Cited by
-
Towards the development of functionalized polypyridine ligands for Ru(II) complexes as photosensitizers in dye-sensitized solar cells (DSSCs).Molecules. 2014 Aug 15;19(8):12421-60. doi: 10.3390/molecules190812421. Molecules. 2014. PMID: 25153864 Free PMC article. Review.
References
-
- Schanze KS, Schmehl RH. Applications of inorganic photochemistry in the chemical and biological sciences—contemporary developments. Journal of Chemical Education. 1997;74(6):633–640.
-
- Miller WW, Yafuso M, Yan CF, Hui HK, Arick S. Performance of an in-vivo, continuous blood-gas monitor with disposable probe. Clinical Chemistry. 1987;33(9):1538–1542. - PubMed
-
- Demas JN, DeGraff BA. Applications of luminescent transition metal complexes to sensor technology and molecular probes. Journal of Chemical Education. 1997;74(6):690–695.
-
- Collins GE, Rose-Pehrsson SL. Chemiluminescent chemical sensors for inorganic and organic vapors. Sensors and Actuators, B: Chemical. 1996;34(1–3):317–322.
-
- Cook MJ, Lewis AP, McAuliffe GSG, et al. Luminescent metal complexes. Part 1. Tris-chelates of substituted 2,2′-bipyridyls with ruthenium(II) as dyes for luminescent solar collectors. Journal of the Chemical Society, Perkin Transactions 2. 1984;(8):1293–1301.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

