Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study
- PMID: 24884381
- PMCID: PMC4035673
- DOI: 10.1186/1471-2288-14-70
Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study
Abstract
Background: The validity of a meta-analysis can be understood better in light of the possible impact of publication bias. The majority of the methods to investigate publication bias in terms of small study-effects are developed for meta-analyses of intervention studies, leaving authors of diagnostic test accuracy (DTA) systematic reviews with limited guidance. The aim of this study was to evaluate if and how publication bias was assessed in meta-analyses of DTA, and to compare the results of various statistical methods used to assess publication bias.
Methods: A systematic search was initiated to identify DTA reviews with a meta-analysis published between September 2011 and January 2012. We extracted all information about publication bias from the reviews and the two-by-two tables. Existing statistical methods for the detection of publication bias were applied on data from the included studies.
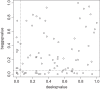
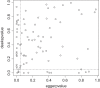
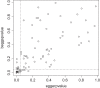
Results: Out of 1,335 references, 114 reviews could be included. Publication bias was explicitly mentioned in 75 reviews (65.8%) and 47 of these had performed statistical methods to investigate publication bias in terms of small study-effects: 6 by drawing funnel plots, 16 by statistical testing and 25 by applying both methods. The applied tests were Egger's test (n = 18), Deeks' test (n = 12), Begg's test (n = 5), both the Egger and Begg tests (n = 4), and other tests (n = 2). Our own comparison of the results of Begg's, Egger's and Deeks' test for 92 meta-analyses indicated that up to 34% of the results did not correspond with one another.
Conclusions: The majority of DTA review authors mention or investigate publication bias. They mainly use suboptimal methods like the Begg and Egger tests that are not developed for DTA meta-analyses. Our comparison of the Begg, Egger and Deeks tests indicated that these tests do give different results and thus are not interchangeable. Deeks' test is recommended for DTA meta-analyses and should be preferred.
Figures
References
-
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7:1–76. - PubMed
-
- Moher D, Fortin P, Jadad AR, Juni P, Klassen T, Le LJ, Liberati A, Linde K, Penna A. Completeness of reporting of trials published in languages other than English: implications for conduct and reporting of systematic reviews. Lancet. 1996;347:363–366. doi: 10.1016/S0140-6736(96)90538-3. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

