Chronic alcohol consumption from adolescence-to-adulthood in mice--hypothalamic gene expression changes in the dilated cardiomyopathy signaling pathway
- PMID: 24884436
- PMCID: PMC4027996
- DOI: 10.1186/1471-2202-15-61
Chronic alcohol consumption from adolescence-to-adulthood in mice--hypothalamic gene expression changes in the dilated cardiomyopathy signaling pathway
Abstract
Background: Adolescence is a developmental stage vulnerable to alcohol drinking-related problems and the onset of alcoholism. Hypothalamus is a key brain region for food and water intake regulation, and is one of the alcohol-sensitive brain regions. However, it is not known what would be the alcohol effect on hypothalamus following adolescent alcohol intake, chronically over the adolescent development, at moderate levels.
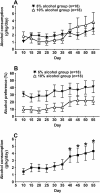
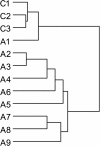
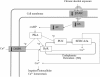
Results: We employed a paradigm of chronic moderate alcohol intake from adolescence-to-adulthood in mice, and analyzed the alcohol effect on both behavioral and hypothalamic gene expression changes. A total of 751 genes were found and subjected to pathway analysis. The dilated cardiomyopathy (DCM) pathway was identified. The changes of ten genes under this pathway were further verified using RT-PCR. Chronic alcohol consumption during adolescence, even at moderate levels, led to a decrease of motor activity in mice, and also a concerted down regulation of signaling pathway initiating factor (SPIF) genes in the DCM signaling pathway, including β1-adrenergic receptor (Adrb1), Gs protein (Gnas), adenylyl cyclase 1 (Adcy1), and dihydropyridine receptor/L-type calcium channel (Cacna1d).
Conclusions: These findings suggest that adolescent alcohol intake may trigger gene expression changes in the CNS that parallel those found in the dilated cardiomyopathy signaling pathway. If such effects also take place in humans, our findings would serve as a warning against alcohol intake in youth, such as by teens and/or college students.
Figures


Similar articles
-
Chronic alcohol consumption from adolescence to adulthood in mice--hypothalamic gene expression changes in insulin-signaling pathway.Alcohol. 2014 Sep;48(6):571-8. doi: 10.1016/j.alcohol.2014.07.001. Epub 2014 Jul 5. Alcohol. 2014. PMID: 25088817
-
Changes in cardiac signal transduction systems in chronic ethanol treatment preceding the development of alcoholic cardiomyopathy.Herz. 1996 Aug;21(4):232-40. Herz. 1996. PMID: 8805003
-
Alcohol consumption and biological markers for alcoholism in idiopathic dilated cardiomyopathy: a case-controlled study.Alcohol Alcohol. 1992 Jul;27(4):353-8. Alcohol Alcohol. 1992. PMID: 1418109
-
Hypothalamic neuropeptide signaling in alcohol addiction.Prog Neuropsychopharmacol Biol Psychiatry. 2016 Feb 4;65:321-9. doi: 10.1016/j.pnpbp.2015.02.006. Epub 2015 Feb 14. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 25689818 Free PMC article. Review.
-
Alcoholic dilated cardiomyopathy.Nurs Stand. 2008 May 28-Jun 3;22(38):42-7. Nurs Stand. 2008. PMID: 18578120 Review.
Cited by
-
Studying alcohol use disorder using Drosophila melanogaster in the era of 'Big Data'.Behav Brain Funct. 2019 Apr 16;15(1):7. doi: 10.1186/s12993-019-0159-x. Behav Brain Funct. 2019. PMID: 30992041 Free PMC article. Review.
-
p39 Affects Myelin Formation in Cerebral Ischemic Injury.Neuromolecular Med. 2024 Jun 1;26(1):22. doi: 10.1007/s12017-024-08792-3. Neuromolecular Med. 2024. PMID: 38824254
-
A perspective profile of ADCY1 in cAMP signaling with drug-resistance in lung cancer.J Cancer. 2019 Nov 1;10(27):6848-6857. doi: 10.7150/jca.36614. eCollection 2019. J Cancer. 2019. PMID: 31839819 Free PMC article. Review.
-
Expression Signatures of Long Noncoding RNAs in Left Ventricular Noncompaction.Front Cardiovasc Med. 2021 Nov 10;8:763858. doi: 10.3389/fcvm.2021.763858. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34859074 Free PMC article.
-
Chronic intermittent ethanol exposure selectively alters the expression of Gα subunit isoforms and RGS subtypes in rat prefrontal cortex.Brain Res. 2017 Oct 1;1672:106-112. doi: 10.1016/j.brainres.2017.07.014. Epub 2017 Jul 21. Brain Res. 2017. PMID: 28736108 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

