Valproate pretreatment protects pancreatic β-cells from palmitate-induced ER stress and apoptosis by inhibiting glycogen synthase kinase-3β
- PMID: 24884462
- PMCID: PMC4084580
- DOI: 10.1186/1423-0127-21-38
Valproate pretreatment protects pancreatic β-cells from palmitate-induced ER stress and apoptosis by inhibiting glycogen synthase kinase-3β
Abstract
Background: Reduction of pancreatic β-cells mass, major secondary to increased β-cells apoptosis, is increasingly recognized as one of the main contributing factors to the pathogenesis of type 2 diabetes (T2D), and saturated free fatty acid palmitate has been shown to induce endoplasmic reticulum (ER) stress that may contribute to promoting β-cells apoptosis. Recent literature suggests that valproate, a diffusely prescribed drug in the treatment of epilepsy and bipolar disorder, can inhibit glycogen synthase kinase-3β (GSK-3β) activity and has cytoprotective effects in neuronal cells and HepG2 cells. Thus, we hypothesized that valproate may protect INS-1 β-cells from palmitate-induced apoptosis via inhibiting GSK-3β.
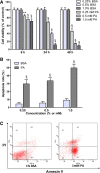
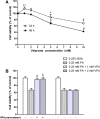
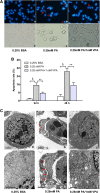
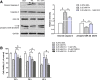
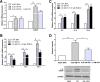
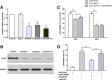
Results: Valproate pretreatment remarkable prevented palmitate-mediated cytotoxicity and apoptosis (lipotoxicity) as well as ER distension. Furthermore, palmitate triggered ER stress as evidenced by increased mRNA levels of C/EBP homologous protein (CHOP) and activating transcription factor 4 (ATF4) in a time-dependent fashion. However, valproate not only reduced the mRNA and protein expression of CHOP but also inhibited GSK-3β and caspase-3 activity induced by palmitate, whereas, the mRNA expression of ATF4 was not affected. Interestingly, TDZD-8, a specific GSK-3β inhibitor, also showed the similar effect on lipotoxicity and ER stress as valproate in INS-1 cells. Finally, compared with CHOP knockdown, valproate displayed better cytoprotection against palmitate.
Conclusions: Valproate may protect β-cells from palmitate-induced apoptosis and ER stress via GSK-3β inhibition, independent of ATF4/CHOP pathway. Besides, GSK-3β, rather than CHOP, may be a more promising therapeutic target for T2D.
Figures
Similar articles
-
Saturated free fatty acid sodium palmitate-induced lipoapoptosis by targeting glycogen synthase kinase-3β activation in human liver cells.Dig Dis Sci. 2014 Feb;59(2):346-57. doi: 10.1007/s10620-013-2896-2. Epub 2013 Oct 17. Dig Dis Sci. 2014. PMID: 24132507
-
Oleate rescues INS-1E β-cells from palmitate-induced apoptosis by preventing activation of the unfolded protein response.Biochem Biophys Res Commun. 2013 Nov 29;441(4):770-6. doi: 10.1016/j.bbrc.2013.10.130. Epub 2013 Nov 1. Biochem Biophys Res Commun. 2013. PMID: 24189472
-
Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling.Diabetes. 2008 Nov;57(11):3034-44. doi: 10.2337/db07-1802. Epub 2008 Jun 30. Diabetes. 2008. PMID: 18591394 Free PMC article.
-
Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection.Bipolar Disord. 2002 Apr;4(2):137-44. doi: 10.1034/j.1399-5618.2002.40201.x. Bipolar Disord. 2002. PMID: 12071511 Free PMC article. Review.
-
Molecular Mechanisms of Apoptosis Induction and Its Regulation by Fatty Acids in Pancreatic β-Cells.Int J Mol Sci. 2021 Apr 20;22(8):4285. doi: 10.3390/ijms22084285. Int J Mol Sci. 2021. PMID: 33924206 Free PMC article. Review.
Cited by
-
Endoplasmic reticulum stress associates with the development of intervertebral disc degeneration.Front Endocrinol (Lausanne). 2023 Jan 12;13:1094394. doi: 10.3389/fendo.2022.1094394. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36714579 Free PMC article. Review.
-
Protein Kinases Signaling in Pancreatic Beta-cells Death and Type 2 Diabetes.Adv Exp Med Biol. 2021;1275:195-227. doi: 10.1007/978-3-030-49844-3_8. Adv Exp Med Biol. 2021. PMID: 33539017
-
Targeting Protein Kinases to Protect Beta-Cell Function and Survival in Diabetes.Int J Mol Sci. 2024 Jun 11;25(12):6425. doi: 10.3390/ijms25126425. Int J Mol Sci. 2024. PMID: 38928130 Free PMC article. Review.
-
GSK-3β mediates dexamethasone-induced pancreatic β cell apoptosis.Life Sci. 2016 Jan 1;144:1-7. doi: 10.1016/j.lfs.2015.11.017. Epub 2015 Nov 25. Life Sci. 2016. PMID: 26606859 Free PMC article.
-
DR5 but not miRNA-181 or miRNA-211 is involved in ER stress-mediated apoptosis induced by palmitate in islet β cells.Int J Clin Exp Pathol. 2017 Jul 1;10(7):7692-7698. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966615 Free PMC article.
References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53:624–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

