A model-based economic analysis of pre-pandemic influenza vaccination cost-effectiveness
- PMID: 24884470
- PMCID: PMC4045999
- DOI: 10.1186/1471-2334-14-266
A model-based economic analysis of pre-pandemic influenza vaccination cost-effectiveness
Abstract
Background: A vaccine matched to a newly emerged pandemic influenza virus would require a production time of at least 6 months with current proven techniques, and so could only be used reactively after the peak of the pandemic. A pre-pandemic vaccine, although probably having lower efficacy, could be produced and used pre-emptively. While several previous studies have investigated the cost effectiveness of pre-emptive vaccination strategies, they have not been directly compared to realistic reactive vaccination strategies.
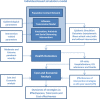
Methods: An individual-based simulation model of ~30,000 people was used to examine a pre-emptive vaccination strategy, assuming vaccination conducted prior to a pandemic using a low-efficacy vaccine. A reactive vaccination strategy, assuming a 6-month delay between pandemic emergence and availability of a high-efficacy vaccine, was also modelled. Social distancing and antiviral interventions were examined in combination with these alternative vaccination strategies. Moderate and severe pandemics were examined, based on estimates of transmissibility and clinical severity of the 1957 and 1918 pandemics respectively, and the cost effectiveness of each strategy was evaluated.
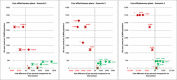
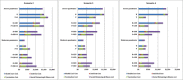
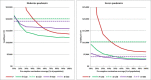
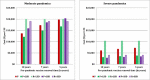
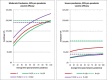
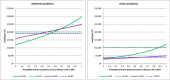
Results: Provided that a pre-pandemic vaccine achieved at least 30% efficacy, pre-emptive vaccination strategies were found to be more cost effective when compared to reactive vaccination strategies. Reactive vaccination coupled with sustained social distancing and antiviral interventions was found to be as effective at saving lives as pre-emptive vaccination coupled with limited duration social distancing and antiviral use, with both strategies saving approximately 420 life-years per 10,000 population for a moderate pandemic with a basic reproduction number of 1.9 and case fatality rate of 0.25%. Reactive vaccination was however more costly due to larger productivity losses incurred by sustained social distancing, costing $8 million per 10,000 population ($19,074/LYS) versus $6.8 million per 10,000 population ($15,897/LYS) for a pre-emptive vaccination strategy. Similar trends were observed for severe pandemics.
Conclusions: Compared to reactive vaccination, pre-emptive strategies would be more effective and more cost effective, conditional on the pre-pandemic vaccine being able to achieve a certain level of coverage and efficacy. Reactive vaccination strategies exist which are as effective at mortality reduction as pre-emptive strategies, though they are less cost effective.
Figures
Similar articles
-
Vaccination strategies for future influenza pandemics: a severity-based cost effectiveness analysis.BMC Infect Dis. 2013 Feb 11;13:81. doi: 10.1186/1471-2334-13-81. BMC Infect Dis. 2013. PMID: 23398722 Free PMC article.
-
The cost effectiveness of pandemic influenza interventions: a pandemic severity based analysis.PLoS One. 2013 Apr 9;8(4):e61504. doi: 10.1371/journal.pone.0061504. Print 2013. PLoS One. 2013. PMID: 23585906 Free PMC article.
-
Economic analysis of pandemic influenza mitigation strategies for five pandemic severity categories.BMC Public Health. 2013 Mar 8;13:211. doi: 10.1186/1471-2458-13-211. BMC Public Health. 2013. PMID: 23496898 Free PMC article.
-
Are we prepared for the next influenza pandemic? Lessons from modelling different preparedness policies against four pandemic scenarios.J Theor Biol. 2019 Nov 21;481:223-232. doi: 10.1016/j.jtbi.2019.05.003. Epub 2019 May 4. J Theor Biol. 2019. PMID: 31059716 Review.
-
Antiviral agents for influenza: a comparison of cost-effectiveness data.Pharmacoeconomics. 2005;23(11):1083-106. doi: 10.2165/00019053-200523110-00003. Pharmacoeconomics. 2005. PMID: 16277546 Review.
Cited by
-
A modelling analysis of the effectiveness of second wave COVID-19 response strategies in Australia.Sci Rep. 2021 Jun 7;11(1):11958. doi: 10.1038/s41598-021-91418-6. Sci Rep. 2021. PMID: 34099788 Free PMC article.
-
Influenza vaccination coverage of health care workers: a cross-sectional study based on data from a Swiss gynaecological hospital.GMS Infect Dis. 2018 Feb 23;6:Doc02. doi: 10.3205/id000037. eCollection 2018. GMS Infect Dis. 2018. PMID: 30671333 Free PMC article.
-
Heterosubtypic protection conferred by the human monoclonal antibody PN-SIA28 against influenza A virus lethal infections in mice.Antimicrob Agents Chemother. 2015 May;59(5):2647-53. doi: 10.1128/AAC.00118-15. Epub 2015 Feb 17. Antimicrob Agents Chemother. 2015. PMID: 25691648 Free PMC article.
-
Complete Protection against Influenza Virus H1N1 Strain A/PR/8/34 Challenge in Mice Immunized with Non-Adjuvanted Novirhabdovirus Vaccines.PLoS One. 2016 Oct 6;11(10):e0164245. doi: 10.1371/journal.pone.0164245. eCollection 2016. PLoS One. 2016. PMID: 27711176 Free PMC article.
-
Refining the approach to vaccines against influenza A viruses with pandemic potential.Future Virol. 2015;10(9):1033-1047. doi: 10.2217/fvl.15.69. Future Virol. 2015. PMID: 26587050 Free PMC article.
References
-
- Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;14(9):687–695. doi: 10.1016/S1473-3099(12)70121-4. - DOI - PubMed
-
- Frost W. Statistics of influenza morbidity with special reference to certain factors in case incidence and case fatality. Public Heath Rep. 1920;14:584–597. doi: 10.2307/4575511. - DOI
-
- Phillip C. Nature outlook: influenza. Nature. 2011;14(7376 Suppl):S1–S15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

