Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma
- PMID: 24884509
- PMCID: PMC4035898
- DOI: 10.1186/1476-4598-13-110
Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma
Abstract
Background: The E3 ubiquitin ligase Fbxw7 functions as a general tumor suppressor by targeting several well-known oncoproteins for ubiquitination and proteasomal degradation. However, the clinical significance of Fbxw7 and the mechanisms involved in the anti-cancer effect of Fbxw7 in HCC are not clear.
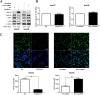
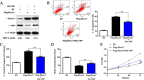
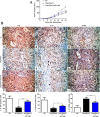
Method: The Fbxw7 and YAP expression in 60 samples of surgical resected HCC and matched normal tumor-adjacent tissues were assessed using IHC or immunoblotting. Flow cytometry, caspase 3/7 activity assay, BrdU cell proliferation assay and MTT assay were used to detect proliferation and apoptosis of HCC cells. The regulatory effect of Fbxw7 on YAP in HCC cells was confirmed by qRT-PCR, immunoblotting and immunofluorescence. Co-immunoprecipitation was used to analyze interaction between YAP and Fbxw7. Nude mice subcutaneous injection, Ki-67 staining and TUNEL assay were used to evaluate tumor growth and apoptosis in vivo.
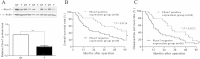
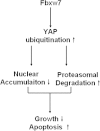
Results: In this study, we found that Fbxw7 expression was impaired in HCC tissues and loss of Fbxw7 expression was correlated with poor clinicopathological features including large tumor size, venous infiltration, high pathological grading and advanced TNM stage. Additionally, we demonstrated that patients with positive Fbxw7 expression had a better 5-year survival and Fbxw7 was an independent factor for predicting the prognosis of HCC patients. We confirmed that Fbxw7 inhibited HCC by inducing both apoptosis and growth arrest. Elevated YAP expression was observed in the same cohort of HCC tissues. Pearson's correlation coefficient analysis indicated that Fbxw7 was inversely associated with YAP protein expression in HCC tissues. We also found that Fbxw7 regulated YAP protein abundance by targeting YAP for ubiquitination and proteasomal degradation in HCC. Furthermore, restoring YAP expression partially abrogated Fbxw7 induced HCC cell apoptosis and growth arrest in vitro and in vivo.
Conclusion: These results indicate that Fbxw7 may serve as a prognostic marker and that YAP may be a potential target of Fbxw7 in HCC.
Figures



Similar articles
-
MicroRNA-92a contributes to tumor growth of human hepatocellular carcinoma by targeting FBXW7.Oncol Rep. 2015 Nov;34(5):2576-84. doi: 10.3892/or.2015.4210. Epub 2015 Aug 19. Oncol Rep. 2015. PMID: 26323375
-
MicroRNA-27b exerts an oncogenic function by targeting Fbxw7 in human hepatocellular carcinoma.Tumour Biol. 2016 Nov;37(11):15325-15332. doi: 10.1007/s13277-016-5444-9. Epub 2016 Oct 4. Tumour Biol. 2016. PMID: 27704356
-
Recombinant human adenovirus-p53 injection induced apoptosis in hepatocellular carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls c-Myc and cyclin E.PLoS One. 2013 Jul 1;8(7):e68574. doi: 10.1371/journal.pone.0068574. Print 2013. PLoS One. 2013. Retraction in: PLoS One. 2020 Mar 27;15(3):e0231287. doi: 10.1371/journal.pone.0231287. PMID: 23840897 Free PMC article. Retracted.
-
The FBXW7-NOTCH interactome: A ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues.Cancer Rep (Hoboken). 2021 Aug;4(4):e1369. doi: 10.1002/cnr2.1369. Epub 2021 Apr 6. Cancer Rep (Hoboken). 2021. PMID: 33822486 Free PMC article. Review.
-
Aberrant regulation of FBW7 in cancer.Oncotarget. 2014 Apr 30;5(8):2000-15. doi: 10.18632/oncotarget.1859. Oncotarget. 2014. PMID: 24899581 Free PMC article. Review.
Cited by
-
Fbxw7 Deletion Accelerates KrasG12D-Driven Pancreatic Tumorigenesis via Yap Accumulation.Neoplasia. 2016 Nov;18(11):666-673. doi: 10.1016/j.neo.2016.08.009. Epub 2016 Oct 18. Neoplasia. 2016. PMID: 27764699 Free PMC article.
-
Inactivation of hypoxia-induced YAP by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells.Sci Rep. 2016 Aug 1;6:30483. doi: 10.1038/srep30483. Sci Rep. 2016. PMID: 27476430 Free PMC article.
-
Regulation of apoptosis by ubiquitination in liver cancer.Am J Cancer Res. 2023 Oct 15;13(10):4832-4871. eCollection 2023. Am J Cancer Res. 2023. PMID: 37970337 Free PMC article. Review.
-
Usp10 Modulates the Hippo Pathway by Deubiquitinating and Stabilizing the Transcriptional Coactivator Yorkie.Int J Mol Sci. 2019 Nov 29;20(23):6013. doi: 10.3390/ijms20236013. Int J Mol Sci. 2019. PMID: 31795326 Free PMC article.
-
SHARPIN Inhibits Esophageal Squamous Cell Carcinoma Progression by Modulating Hippo Signaling.Neoplasia. 2020 Feb;22(2):76-85. doi: 10.1016/j.neo.2019.12.001. Epub 2019 Dec 26. Neoplasia. 2020. PMID: 31884247 Free PMC article.
References
-
- Tu K, Zheng X, Yin G, Zan X, Yao Y, Liu Q. Evaluation of Fbxw7 expression and its correlation with expression of SREBP-1 in a mouse model of NAFLD. Mol Med Rep. 2012;6:525–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

