Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism
- PMID: 24884523
- PMCID: PMC4047260
- DOI: 10.1186/1476-4598-13-130
Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism
Expression of concern in
-
Editorial expression of concern: Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism.Mol Cancer. 2025 Apr 14;24(1):112. doi: 10.1186/s12943-025-02326-6. Mol Cancer. 2025. PMID: 40223104 Free PMC article. No abstract available.
Abstract
Background: Increasing evidence indicates an important role of transcription factor Yin Yang-1 (YY1) in human tumorigenesis. However, its function in cancer remains controversial and the relevance of YY1 to pancreatic ductal adenocarcinoma (PDAC) remains to be clarified.
Methods: In this study, we detected YY1 expression in clinical PDAC tissue samples and cell lines using quantitative RT-PCR, immunohistochemistry and western blotting. We also detected MUC4 and MMP10 mRNA levels in 108 PDAC samples using qRT-PCR and analyzed the correlations between YY1 and MUC4 or MMP10 expression. The role of YY1 in the proliferation, invasion and metastatic abilities of PDAC cells in vitro was studied by CCK-8 assay, cell migration and invasion assays. In vivo pancreatic tumor growth and metastasis was studied by a xenogenous subcutaneously implant model and a tail vein metastasis model. The potential mechanisms underlying YY1 mediated tumor progression in PDAC were explored by digital gene expression (DGE) sequencing, signal transduction pathways blockage experiments and luciferase assays. Statistical analysis was performed using the SPSS 15.0 software.
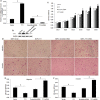
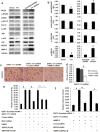
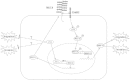
Results: We found that the expression of YY1 in PDACs was higher compared with their adjacent non-tumorous tissues and normal pancreas tissues. However, PDAC patients with high level overexpression of YY1 had better outcome than those with low level overexpression. YY1 expression levels were statistically negatively correlated with MMP10 expression levels, but not correlated with MUC4 expression levels. YY1 overexpression suppressed, whereas YY1 knockdown enhanced, the proliferation, invasion and metastatic properties of BXPC-3 cells, both in vitro and in vivo. YY1 suppresses invasion and metastasis of pancreatic cancer cells by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism.
Conclusions: The present study suggested that YY1 plays a negative role, i.e. is a tumor suppressor, in PDAC, and may become a valuable diagnostic and prognostic marker of PDAC.
Figures



Similar articles
-
Yin Yang-1 suppresses pancreatic ductal adenocarcinoma cell proliferation and tumor growth by regulating SOX2OT-SOX2 axis.Cancer Lett. 2017 Nov 1;408:144-154. doi: 10.1016/j.canlet.2017.08.032. Epub 2017 Sep 1. Cancer Lett. 2017. PMID: 28867247
-
YY1 suppresses proliferation and migration of pancreatic ductal adenocarcinoma by regulating the CDKN3/MdM2/P53/P21 signaling pathway.Int J Cancer. 2018 Apr 1;142(7):1392-1404. doi: 10.1002/ijc.31173. Epub 2017 Dec 4. Int J Cancer. 2018. Retraction in: Int J Cancer. 2024 Mar 1;154(5):E5. doi: 10.1002/ijc.34803. PMID: 29168185 Retracted.
-
Knockdown of FOXO3a induces epithelial-mesenchymal transition and promotes metastasis of pancreatic ductal adenocarcinoma by activation of the β-catenin/TCF4 pathway through SPRY2.J Exp Clin Cancer Res. 2019 Jan 28;38(1):38. doi: 10.1186/s13046-019-1046-x. J Exp Clin Cancer Res. 2019. Retraction in: J Exp Clin Cancer Res. 2022 Jun 17;41(1):204. doi: 10.1186/s13046-022-02413-2. PMID: 30691517 Free PMC article. Retracted.
-
Role of YY1 in pancreatic ductal adenocarcinoma: Mechanisms and therapeutic perspectives.Biochim Biophys Acta Rev Cancer. 2025 Aug 9;1880(5):189415. doi: 10.1016/j.bbcan.2025.189415. Online ahead of print. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40789430 Review.
-
The roles of YY1 in reproductive system: an overview.Chem Biol Interact. 2025 Aug 25;417:111560. doi: 10.1016/j.cbi.2025.111560. Epub 2025 May 15. Chem Biol Interact. 2025. PMID: 40381670 Review.
Cited by
-
A phase I/II study of ivaltinostat combined with gemcitabine and erlotinib in patients with untreated locally advanced or metastatic pancreatic adenocarcinoma.Int J Cancer. 2022 Nov 1;151(9):1565-1577. doi: 10.1002/ijc.34144. Epub 2022 Jun 21. Int J Cancer. 2022. PMID: 35657348 Free PMC article. Clinical Trial.
-
Methylation‑associated silencing of miR‑638 promotes endometrial carcinoma progression by targeting MEF2C.Int J Mol Med. 2020 Jun;45(6):1753-1770. doi: 10.3892/ijmm.2020.4540. Epub 2020 Mar 16. Int J Mol Med. 2020. PMID: 32186750 Free PMC article.
-
Geldanamycin treatment does not result in anti-cancer activity in a preclinical model of orthotopic mesothelioma.PLoS One. 2023 May 5;18(5):e0274364. doi: 10.1371/journal.pone.0274364. eCollection 2023. PLoS One. 2023. PMID: 37146029 Free PMC article.
-
Human Papillomavirus Infection in Head and Neck Squamous Cell Carcinomas: Transcriptional Triggers and Changed Disease Patterns.Front Cell Infect Microbiol. 2020 Dec 2;10:537650. doi: 10.3389/fcimb.2020.537650. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33344262 Free PMC article. Review.
-
MEF2A, MEF2C, and MEF2D as potential biomarkers of pancreatic cancer?BMC Cancer. 2025 Apr 25;25(1):775. doi: 10.1186/s12885-025-14107-x. BMC Cancer. 2025. PMID: 40281485 Free PMC article.
References
-
- Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochim Biophys Acta. 1997;1332:F49–F66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

