Natural transformation of Thermotoga sp. strain RQ7
- PMID: 24884561
- PMCID: PMC4029938
- DOI: 10.1186/1472-6750-14-39
Natural transformation of Thermotoga sp. strain RQ7
Abstract
Background: Thermotoga species are organisms of enormous interest from a biotechnological as well as evolutionary point of view. Genetic modifications of Thermotoga spp. are often desired in order to fully release their multifarious potentials. Effective transformation of recombinant DNA into these bacteria constitutes a critical step of such efforts. This study aims to establish natural competency in Thermotoga spp. and to provide a convenient method to transform these organisms.
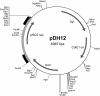
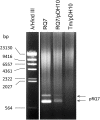
Results: Foreign DNA was found to be relatively stable in the supernatant of a Thermotoga culture for up to 6 hours. Adding donor DNA to T. sp. strain RQ7 at its early exponential growth phase (OD600 0.18 ~ 0.20) resulted in direct acquisition of the DNA by the cells. Both T. neapolitana chromosomal DNA and Thermotoga-E. coli shuttle vectors effectively transformed T. sp. strain RQ7, rendering the cells resistance to kanamycin. The kan gene carried by the shuttle vector pDH10 was detected by PCR from the plasmid extract of the transformants, and the amplicons were verified by restriction digestions. A procedure for natural transformation of Thermotoga spp. was established and optimized. With the optimized method, T. sp. strain RQ7 sustained a transformation frequency in the order of 10⁻⁷ with both genomic and plasmid DNA.
Conclusions: T. sp. strain RQ7 cells are naturally transformable during their early exponential phase. They acquire DNA from both closely and distantly related species. Both chromosomal DNA and plasmid DNA serve as suitable substrates for transformation. Our findings lend a convenient technical tool for the genetic engineering of Thermotoga spp.
Figures


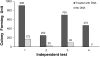


Similar articles
-
Construction and transformation of a Thermotoga-E. coli shuttle vector.BMC Biotechnol. 2012 Jan 6;12:2. doi: 10.1186/1472-6750-12-2. BMC Biotechnol. 2012. PMID: 22225774 Free PMC article.
-
Development of a pyrE-based selective system for Thermotoga sp. strain RQ7.Extremophiles. 2017 Mar;21(2):297-306. doi: 10.1007/s00792-016-0902-2. Epub 2016 Dec 7. Extremophiles. 2017. PMID: 27928679
-
Liposome-mediated DNA uptake and transient expression in Thermotoga.Extremophiles. 2001 Feb;5(1):53-60. doi: 10.1007/s007920000173. Extremophiles. 2001. PMID: 11302503
-
Plasmid pRQ7 from the hyperthermophilic bacterium Thermotoga species strain RQ7 replicates by the rolling-circle mechanism.J Bacteriol. 1997 Nov;179(22):7161-4. doi: 10.1128/jb.179.22.7161-7164.1997. J Bacteriol. 1997. PMID: 9371465 Free PMC article.
-
[Advances in the molecular mechanism of natural bacterial transformation--a review].Wei Sheng Wu Xue Bao. 2012 Jan;52(1):6-11. Wei Sheng Wu Xue Bao. 2012. PMID: 22489454 Review. Chinese.
Cited by
-
Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals.Front Microbiol. 2015 Nov 5;6:1209. doi: 10.3389/fmicb.2015.01209. eCollection 2015. Front Microbiol. 2015. PMID: 26594201 Free PMC article. Review.
-
Occurrence of Capnophilic Lactic Fermentation in the Hyperthermophilic Anaerobic Bacterium Thermotoga sp. Strain RQ7.Int J Mol Sci. 2022 Oct 10;23(19):12049. doi: 10.3390/ijms231912049. Int J Mol Sci. 2022. PMID: 36233345 Free PMC article.
-
Complete genome sequence of Thermotoga sp. strain RQ7.Stand Genomic Sci. 2017 Oct 11;12:62. doi: 10.1186/s40793-017-0271-1. eCollection 2017. Stand Genomic Sci. 2017. PMID: 29046741 Free PMC article.
-
Adapted laboratory evolution of Thermotoga sp. strain RQ7 under carbon starvation.BMC Res Notes. 2022 Mar 10;15(1):99. doi: 10.1186/s13104-022-05982-9. BMC Res Notes. 2022. PMID: 35272671 Free PMC article.
-
Improvement of CO2 and Acetate Coupling into Lactic Acid by Genetic Manipulation of the Hyperthermophilic Bacterium Thermotoga neapolitana.Microorganisms. 2021 Aug 9;9(8):1688. doi: 10.3390/microorganisms9081688. Microorganisms. 2021. PMID: 34442767 Free PMC article.
References
-
- Schroder C, Selig M, Schonheit P. Glucose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic eubacterium thermotoga maritima: involvement of the embden-meyerhof pathway. Arch Microbiol. 1994;161(6):460–470.
-
- Selig M, Xavier KB, Santos H, Schonheit P. Comparative analysis of embden-meyerhof and entner-doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium thermotoga. Arch Microbiol. 1997;167(4):217–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

