Dynamics of a combined Medea-underdominant population transformation system
- PMID: 24884575
- PMCID: PMC4068157
- DOI: 10.1186/1471-2148-14-98
Dynamics of a combined Medea-underdominant population transformation system
Abstract
Background: Transgenic constructs intended to be stably established at high frequencies in wild populations have been demonstrated to "drive" from low frequencies in experimental insect populations. Linking such population transformation constructs to genes which render them unable to transmit pathogens could eventually be used to stop the spread of vector-borne diseases like malaria and dengue.
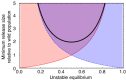
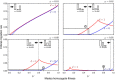
Results: Generally, population transformation constructs with only a single transgenic drive mechanism have been envisioned. Using a theoretical modelling approach we describe the predicted properties of a construct combining autosomal Medea and underdominant population transformation systems. We show that when combined they can exhibit synergistic properties which in broad circumstances surpass those of the single systems.
Conclusion: With combined systems, intentional population transformation and its reversal can be achieved readily. Combined constructs also enhance the capacity to geographically restrict transgenic constructs to targeted populations. It is anticipated that these properties are likely to be of particular value in attracting regulatory approval and public acceptance of this novel technology.
Figures





Similar articles
-
Inverse Medea as a novel gene drive system for local population replacement: a theoretical analysis.J Hered. 2011 May-Jun;102(3):336-41. doi: 10.1093/jhered/esr019. J Hered. 2011. PMID: 21493596 Free PMC article.
-
Medea selfish genetic elements as tools for altering traits of wild populations: a theoretical analysis.Evolution. 2011 Apr;65(4):1149-62. doi: 10.1111/j.1558-5646.2010.01186.x. Epub 2010 Dec 22. Evolution. 2011. PMID: 21062278 Free PMC article.
-
Modeling the dynamics of a non-limited and a self-limited gene drive system in structured Aedes aegypti populations.PLoS One. 2013 Dec 10;8(12):e83354. doi: 10.1371/journal.pone.0083354. eCollection 2013. PLoS One. 2013. PMID: 24340097 Free PMC article.
-
Gene drive systems in mosquitoes: rules of the road.Trends Parasitol. 2005 Feb;21(2):64-7. doi: 10.1016/j.pt.2004.11.004. Trends Parasitol. 2005. PMID: 15664528 Review.
-
Gene drive systems for insect disease vectors.Nat Rev Genet. 2006 Jun;7(6):427-35. doi: 10.1038/nrg1870. Nat Rev Genet. 2006. PMID: 16682981 Review.
Cited by
-
CRISPR/Cas9 Gene Drive: Growing Pains for a New Technology.Genetics. 2017 Mar;205(3):1037-1039. doi: 10.1534/genetics.116.198887. Genetics. 2017. PMID: 28270527 Free PMC article. No abstract available.
-
Split versions of Cleave and Rescue selfish genetic elements for measured self limiting gene drive.PLoS Genet. 2021 Feb 18;17(2):e1009385. doi: 10.1371/journal.pgen.1009385. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33600432 Free PMC article.
-
Cheating evolution: engineering gene drives to manipulate the fate of wild populations.Nat Rev Genet. 2016 Mar;17(3):146-59. doi: 10.1038/nrg.2015.34. Epub 2016 Feb 15. Nat Rev Genet. 2016. PMID: 26875679 Review.
-
A common gene drive language eases regulatory process and eco-evolutionary extensions.BMC Ecol Evol. 2021 Aug 9;21(1):156. doi: 10.1186/s12862-021-01881-y. BMC Ecol Evol. 2021. PMID: 34372763 Free PMC article.
-
Reflection on the Challenges, Accomplishments, and New Frontiers of Gene Drives.Biodes Res. 2022 Aug 6;2022:9853416. doi: 10.34133/2022/9853416. eCollection 2022. Biodes Res. 2022. PMID: 37850135 Free PMC article. Review.
References
-
- Jasinskiene N, Coleman J, Ashikyan A, Salampessy M, Marinotti O. Genetic control of malaria parasite transmission: threshold levels for infection in an avian model system. Am J Trop Med Hyg. 2007;76:1072–1078. - PubMed
-
- Corby-Harris V, Drexler A, Watkins de Jong L, Antonova Y, Pakpour N, Ziegler R, Ramberg F, Lewis E, Brown JM, Luckhart S, Riehle MA. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010;6:1001003. doi: 10.1371/journal.ppat.1001003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

