A mechanically-induced colon cancer cell population shows increased metastatic potential
- PMID: 24884630
- PMCID: PMC4072622
- DOI: 10.1186/1476-4598-13-131
A mechanically-induced colon cancer cell population shows increased metastatic potential
Abstract
Background: Metastasis accounts for the majority of deaths from cancer. Although tumor microenvironment has been shown to have a significant impact on the initiation and/or promotion of metastasis, the mechanism remains elusive. We previously reported that HCT-8 colon cancer cells underwent a phenotypic transition from an adhesive epithelial type (E-cell) to a rounded dissociated type (R-cell) via soft substrate culture, which resembled the initiation of metastasis. The objective of current study was to investigate the molecular and metabolic mechanisms of the E-R transition.
Methods: Global gene expressions of HCT-8 E and R cells were measured by RNA Sequencing (RNA-seq); and the results were further confirmed by real-time PCR. Reactive oxygen species (ROS), anoikis resistance, enzyme activity of aldehyde dehydrogenase 3 family, member A1 (ALDH3A1), and in vitro invasion assay were tested on both E and R cells. The deformability of HCT-8 E and R cells was measured by atomic force microscopy (AFM). To study the in vivo invasiveness of two cell types, athymic nude mice were intra-splenically injected with HCT-8 E or R cells and sacrificed after 9 weeks. Incidences of tumor development and metastasis were histologically evaluated and analyzed with Fisher's exact test.
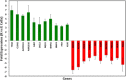
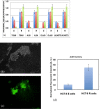
Results: Besides HCT-8, E-R transition on soft substrates was also seen in three other cancer cell lines (HCT116, SW480 colon and DU145 prostate cancer). The expression of some genes, such as ALDH3A1, TNS4, CLDN2, and AKR1B10, which are known to play important roles in cancer cell migration, invasion, proliferation and apoptosis, were increased in HCT-8 R cells. R cells also showed higher ALDH3A1 enzyme activity, higher ROS, higher anoikis resistance, and higher softness than E cells. More importantly, in vitro assay and in vivo animal models revealed that HCT-8 R cells were more invasive than E cells.
Conclusions: Our comprehensive comparison of HCT-8 E and R cells revealed differences of molecular, phenotypical, and mechanical signatures between the two cell types. To our knowledge, this is the first study that explores the molecular mechanism of E-R transition, which may greatly increase our understanding of the mechanisms of cancer mechanical microenvironment and initiation of cancer metastasis.
Figures



Similar articles
-
Pea3 expression promotes the invasive and metastatic potential of colorectal carcinoma.World J Gastroenterol. 2014 Dec 14;20(46):17376-87. doi: 10.3748/wjg.v20.i46.17376. World J Gastroenterol. 2014. PMID: 25516649 Free PMC article.
-
Aldo-keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: Implication for cancer intervention.Int J Cancer. 2007 Nov 15;121(10):2301-6. doi: 10.1002/ijc.22933. Int J Cancer. 2007. PMID: 17597105
-
TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition.Oncogene. 2008 Apr 17;27(18):2635-47. doi: 10.1038/sj.onc.1210914. Epub 2007 Oct 29. Oncogene. 2008. PMID: 17968309
-
Fibrates in the chemical action of daunorubicin.Curr Cancer Drug Targets. 2009 May;9(3):366-9. doi: 10.2174/156800909788166538. Curr Cancer Drug Targets. 2009. PMID: 19442055 Free PMC article. Review.
-
Linking cell mechanical memory and cancer metastasis.Nat Rev Cancer. 2024 Mar;24(3):216-228. doi: 10.1038/s41568-023-00656-5. Epub 2024 Jan 18. Nat Rev Cancer. 2024. PMID: 38238471 Free PMC article. Review.
Cited by
-
Opposing roles of the aldo-keto reductases AKR1B1 and AKR1B10 in colorectal cancer.Cell Oncol (Dordr). 2017 Dec;40(6):563-578. doi: 10.1007/s13402-017-0351-7. Epub 2017 Sep 19. Cell Oncol (Dordr). 2017. PMID: 28929377
-
Decoupling chemical and mechanical signaling in colorectal cancer cell migration.Sci Rep. 2025 Feb 10;15(1):4952. doi: 10.1038/s41598-025-89152-4. Sci Rep. 2025. PMID: 39929899 Free PMC article.
-
Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling.Sci Rep. 2020 Jan 9;10(1):50. doi: 10.1038/s41598-019-55687-6. Sci Rep. 2020. PMID: 31919369 Free PMC article.
-
Decellularized Colorectal Cancer Matrices as Bioactive Scaffolds for Studying Tumor-Stroma Interactions.Cancers (Basel). 2022 Jan 12;14(2):359. doi: 10.3390/cancers14020359. Cancers (Basel). 2022. PMID: 35053521 Free PMC article. Review.
-
Biophysics Role and Biomimetic Culture Systems of ECM Stiffness in Cancer EMT.Glob Chall. 2022 Mar 20;6(6):2100094. doi: 10.1002/gch2.202100094. eCollection 2022 Jun. Glob Chall. 2022. PMID: 35712024 Free PMC article. Review.
References
-
- Weinberg RA. The Biology of Cancer. New York: Garland Science; 2007.
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

