A phase ΙI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study)
- PMID: 24884643
- PMCID: PMC4021539
- DOI: 10.1186/1479-5876-12-108
A phase ΙI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study)
Abstract
Background: We previously conducted a phase I trial for advanced colorectal cancer (CRC) using five HLA-A*2402-restricted peptides, three derived from oncoantigens and two from vascular endothelial growth factor (VEGF) receptors, and confirmed safety and immunological responses. To evaluate clinical benefits of cancer vaccination treatment, we conducted a phase II trial using the same peptides in combination with oxaliplatin-based chemotherapy as a first-line therapy.
Methods: The primary objective of the study was the response rates (RR). Progression free survival (PFS), overall survival (OS), and immunological parameters were evaluated as secondary objective. The planned sample size was more than 40 patients for both HLA2402-matched and -unmatched groups. All patients received a cocktail of five peptides (3 mg each) mixed with 1.5 ml of IFA which was subcutaneously administered weekly for the first 12 weeks followed by biweekly administration. Presence or absence of the HLA-A*2402 genotype were used for classification of patients into two groups.
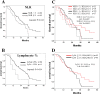
Results: Between February 2009 and November 2012, ninety-six chemotherapy naïve CRC patients were enrolled under the masking of their HLA-A status. Ninety-three patients received mFOLFOX6 and three received XELOX. Bevacizumab was added in five patients. RR was 62.0% and 60.9% in the HLA-A*2402-matched and -unmatched groups, respectively (p=0.910). The median OS was 20.7 months in the HLA-A*2402-matched group and 24.0 months in the unmatched group (log-rank, p=0.489). In subgroup with a neutrophil/lymphocyte ratio (NLR) of <3.0, patients in the HLA-matched group did not survive significantly longer than those in the unmatched group (log-rank, p=0.289) but showed a delayed response.
Conclusions: Although no significance was observed for planned statistical efficacy endpoints, a delayed response was observed in subgroup with a NLR of <3.0. Biomarkers such as NLR might be useful for selecting patients with a better treatment outcome by the vaccination.
Trial registration: Trial registration: UMIN000001791.
Figures


References
-
- Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. - PubMed
-
- Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. - DOI - PubMed
-
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. - DOI - PubMed
-
- Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

