CBD binding domain fused γ-lactamase from Sulfolobus solfataricus is an efficient catalyst for (-) γ-lactam production
- PMID: 24884655
- PMCID: PMC4041915
- DOI: 10.1186/1472-6750-14-40
CBD binding domain fused γ-lactamase from Sulfolobus solfataricus is an efficient catalyst for (-) γ-lactam production
Abstract
Background: γ-lactamase is used for the resolution of γ-lactam which is utilized in the synthesizing of abacavir and peramivir. In some cases, enzymatic method is the most utilized method because of its high efficiency and productivity. The cellulose binding domain (CBD) of cellulose is often used as the bio-specific affinity matrix for enzyme immobilization. Cellulose is cheap and it has excellent chemical and physical properties. Meanwhile, binding between cellulose and CBD is tight and the desorption rarely happened.
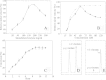
Results: We prepared two fusion constructs of the γ-lactamase gene gla, which was from Sulfolobus solfataricus P2. These two constructs had Cbd (cellulose binding domain from Clostridium thermocellum) fused at amino or carboxyl terminus of the γ-lactamase. These two constructs were heterogeneously expressed in E. coli rosetta (DE3) as two fusion proteins. Both of them were immobilized well on Avicel (microcrystalline cellulose matrix). The apparent kinetic parameters revealed that carboxyl terminus fused protein (Gla-linker-Cbd) was a better catalyst. The V(max) and k(cat) value of Avicel immobilized Gla-linker-Cbd were 381 U mg⁻¹ and 4.7 × 10⁵ s⁻¹ respectively. And the values of the free Gla-linker-Cbd were 151 U mg⁻¹ and 1.8 × 10⁵ s⁻¹ respectively. These data indicated that the catalytic efficiency of the enzyme was upgraded after immobilization. The immobilized Gla-linker-Cbd had a 10-degree temperature optimum dropping from 80°C to 70°C but it was stable when incubated at 60°C for 48 h. It remained stable in catalyzing 20-batch reactions. After optimization, the immobilized enzyme concentration in transformation was set as 200 mg/mL. We found out that there was inhibition that occurred to the immobilized enzyme when substrate concentration exceeded 60 mM. Finally a 10 mL-volume transformation was conducted, in which 0.6 M substrate was hydrolyzed and the resolution was completed within 9 h with a 99.5% ee value.
Conclusions: Cellulose is the most abundant and renewable material on the Earth. The absorption between Cbd domain and cellulose is a bio-green process. The cellulose immobilized fusion Gla exhibited good catalytic characters, therefore we think the cellulose immobilized Gla is a promising catalyst for the industrial preparation of (-) - γ-lactam.
Figures


Similar articles
-
Dynamic kinetic resolution of Vince lactam catalyzed by γ-lactamases: a mini-review.J Ind Microbiol Biotechnol. 2018 Dec;45(12):1017-1031. doi: 10.1007/s10295-018-2093-6. Epub 2018 Oct 23. J Ind Microbiol Biotechnol. 2018. PMID: 30353294 Review.
-
Autodisplay of an archaeal γ-lactamase on the cell surface of Escherichia coli using Xcc_Est as an anchoring scaffold and its application for preparation of the enantiopure antiviral drug intermediate (-) vince lactam.Appl Microbiol Biotechnol. 2014 Aug;98(16):6991-7001. doi: 10.1007/s00253-014-5704-9. Epub 2014 Apr 23. Appl Microbiol Biotechnol. 2014. PMID: 24756321
-
[Analysis of immobilized L-glutamate oxidase fused with cellulose binding domain on microcrystalline cellulose].Sheng Wu Gong Cheng Xue Bao. 2016 Oct 25;32(10):1348-1361. doi: 10.13345/j.cjb.160084. Sheng Wu Gong Cheng Xue Bao. 2016. PMID: 29027445 Chinese.
-
Expression, immobilization and enzymatic properties of glutamate decarboxylase fused to a cellulose-binding domain.Int J Mol Sci. 2012;13(1):358-68. doi: 10.3390/ijms13010358. Epub 2011 Dec 28. Int J Mol Sci. 2012. PMID: 22312257 Free PMC article.
-
[Advances in lactamases from microbes--a review].Wei Sheng Wu Xue Bao. 2010 Aug;50(8):988-94. Wei Sheng Wu Xue Bao. 2010. PMID: 20931864 Review. Chinese.
Cited by
-
Substrate inhibition by the blockage of product release and its control by tunnel engineering.RSC Chem Biol. 2021 Jan 11;2(2):645-655. doi: 10.1039/d0cb00171f. eCollection 2021 Apr 1. RSC Chem Biol. 2021. PMID: 34458806 Free PMC article.
-
Light induced expression of β-glucosidase in Escherichia coli with autolysis of cell.BMC Biotechnol. 2017 Nov 7;17(1):74. doi: 10.1186/s12896-017-0402-1. BMC Biotechnol. 2017. PMID: 29115967 Free PMC article.
-
Dynamic kinetic resolution of Vince lactam catalyzed by γ-lactamases: a mini-review.J Ind Microbiol Biotechnol. 2018 Dec;45(12):1017-1031. doi: 10.1007/s10295-018-2093-6. Epub 2018 Oct 23. J Ind Microbiol Biotechnol. 2018. PMID: 30353294 Review.
References
-
- Toogood HS, Brown RC, Line K, Keene PA, Taylor SJC, McCague R, Littlechild JA. The use of a thermostable signature amidase in the resolution of the bicyclic synthon (rac)-gamma-lactam. Tetrahedron. 2004;60(3):711–716. doi: 10.1016/j.tet.2003.11.064. - DOI
-
- Wang J, Zhang X, Min C, Wu S, Zheng G. Single-step purification and immobilization of γ-lactamase and on-column transformation of 2-azabicyclo [2.2.1] hept-5-en-3-one. Process Biochem. 2011;46(1):81–87. doi: 10.1016/j.procbio.2010.07.018. - DOI
-
- Vince R, Hua M. Synthesis of Carbovir and Abacavir from a Carbocyclic Precursor. In: Current Protocols in Nucleic Acid Chemistry. John Wiley & Sons, Inc.; 2001. - PubMed
-
- Gubareva LV, Webster RG, Hayden FG. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother. 2001;45(12):3403–3408. doi: 10.1128/AAC.45.12.3403-3408.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

