Synthesis and activity of three new trinuclear platinums with cis-geometry for terminal metal centres
- PMID: 24884683
- PMCID: PMC4022405
- DOI: 10.1186/1423-0127-21-41
Synthesis and activity of three new trinuclear platinums with cis-geometry for terminal metal centres
Abstract
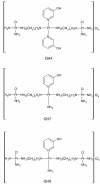
Background: As compared to cisplatin, trinuclear platinum compounds such as BBR3464 and DH6Cl have an altered spectrum of activity possibly because they form long-range adducts with DNA as against mainly intrastrand 1,2-bifunctional adducts formed by cisplatin and its analogues. Because of the labilizing effect associated with the trans-geometry, the compounds are expected to break down inside the cell thus serving to reduce the number of long-range adducts formed. In contrast, trinuclear platinum complexes with cis-geometry for the terminal metal centres would be less subject to such breakdown and hence may produce a greater number of long-range inter- and intrastrand adducts with the DNA. This paper describes the synthesis and activity against human ovarian tumour models of of three new trinuclear platinum complexes with cis-geometry for terminal platinum centres, coded as QH4, QH7 and QH8. The paper also describes cellular accumulation of platinum, level of drug-DNA binding, and nature of interaction of the compounds with pBR322 plasmid DNA.
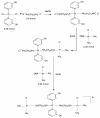
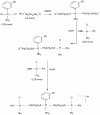
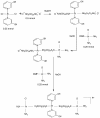
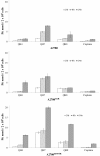
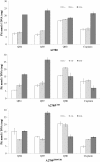
Results: Methods of synthesis, elemental analysis, spectral studies and molar conductivity measurements provide support to the suggested structures of the compounds. QH4 and QH8 are found to be more cytotoxic than cisplatin against the parental A2780 cell line; QH8 is more active than cisplatin against the resistant A2780cisR and A2780ZD0473R cell lines as well. The least compound QH7 shows a greater activity against the resistant cell lines than the parental cell line; it is most damaging to pBR322 plasmid DNA and most able to induce changes in DNA conformation. The variations in activity of the compounds, changes in intracellular drug accumulation and levels of Pt-DNA binding with the changes in number of planaramine ligands bound to central platinum and the length of the linking diamines, can be seen (1) to illustrate structure-activity relationships and (2) to highlight that the relationship between antitumour activity and interaction with cellular platinophiles including DNA can be quite complex as the cell death is carried out by downstream processes in the cell cycle where many proteins are involved.
Conclusion: Among the three designed trinuclear platinum complexes with cis-geometry for the terminal metal centres, the most active compound QH8 is found to be more active than cisplatin against the parental A2780 and the resistant A2780cisR and A2780ZD0473R cell lines.
Figures


Similar articles
-
Synthesis of trans-(4-hydroxypyridine)(ammine)dichloroplatinum(II) and its activity in the human ovarian tumour models.Med Chem. 2013 Jun 1;9(4):539-44. doi: 10.2174/1573406411309040007. Med Chem. 2013. PMID: 23061563
-
Synergism from combination of cisplatin and multicentred platinums coded as DH6Cl and TH1 in the human ovarian tumour models.Med Chem. 2011 Nov;7(6):593-8. doi: 10.2174/157340611797928316. Med Chem. 2011. PMID: 22313299
-
Synthesis and antitumour activity of a new trinuclear platinum compound [{cis-PtCl(NH3)2μ {trans-Pt(3-hydroxypyridine)2H2N(CH2)5NH2)2}] Cl4 in human ovarian cancer cells.Anticancer Res. 2014 Apr;34(4):1923-9. Anticancer Res. 2014. PMID: 24692727
-
Mini-review: discovery and development of platinum complexes designed to circumvent cisplatin resistance.J Inorg Biochem. 1999 Oct;77(1-2):111-5. J Inorg Biochem. 1999. PMID: 10626362 Review.
-
Trans-platinum complexes in cancer therapy.Anticancer Agents Med Chem. 2007 Jan;7(1):111-23. doi: 10.2174/187152007779314080. Anticancer Agents Med Chem. 2007. PMID: 17266508 Review.
References
-
- Stathopoulos GP, Rigatos S, Malamos NA. Paclitaxel combined with cisplatin as second-line treatment in patients with advanced non-small cell lung cancers refractory to cisplatin. Oncol Rep. 1999;6:797–800. - PubMed
-
- Rosenberg B. Noble metal complexes in cancer chemotherapy. Adv Exp Med Biol. 1977;91:129–150. - PubMed
-
- Hill JM, Speer RJ. Organo-platinum complexes as antitumor agents (review) Anticancer Res. 1982;2:173–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

