Combined targeting of TGF-β1 and integrin β3 impairs lymph node metastasis in a mouse model of non-small-cell lung cancer
- PMID: 24884715
- PMCID: PMC4049383
- DOI: 10.1186/1476-4598-13-112
Combined targeting of TGF-β1 and integrin β3 impairs lymph node metastasis in a mouse model of non-small-cell lung cancer
Abstract
Background: Transforming Growth Factor beta (TGF-β) acts as a tumor suppressor early in carcinogenesis but turns into tumor promoter in later disease stages. In fact, TGF-β is a known inducer of integrin expression by tumor cells which contributes to cancer metastatic spread and TGF-β inhibition has been shown to attenuate metastasis in mouse models. However, carcinoma cells often become refractory to TGF-β-mediated growth inhibition. Therefore identifying patients that may benefit from anti-TGF-β therapy requires careful selection.
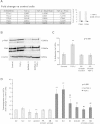
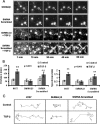
Methods: We performed in vitro analysis of the effects of exposure to TGF-β in NSCLC cell chemotaxis and adhesion to lymphatic endothelial cells. We also studied in an orthotopic model of NSCLC the incidence of metastases to the lymph nodes after inhibition of TGF-β signaling, β3 integrin expression or both.
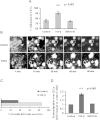
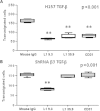
Results: We offer evidences of increased β3-integrin dependent NSCLC adhesion to lymphatic endothelium after TGF-β exposure. In vivo experiments show that targeting of TGF-β and β3 integrin significantly reduces the incidence of lymph node metastasis. Even more, blockade of β3 integrin expression in tumors that did not respond to TGF-β inhibition severely impaired the ability of the tumor to metastasize towards the lymph nodes.
Conclusion: These findings suggest that lung cancer tumors refractory to TGF-β monotherapy can be effectively treated using dual therapy that combines the inhibition of tumor cell adhesion to lymphatic vessels with stromal TGF-β inhibition.
Figures

Similar articles
-
Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β.Mol Biol Cell. 2013 Nov;24(21):3449-59. doi: 10.1091/mbc.E12-10-0776. Epub 2013 Sep 4. Mol Biol Cell. 2013. PMID: 24006485 Free PMC article.
-
Cetuximab-Immunoliposomes Loaded with TGF-β1 siRNA for the Targeting Therapy of NSCLC: Design, and In Vitro and In Vivo Evaluation.Int J Mol Sci. 2025 Jan 30;26(3):1196. doi: 10.3390/ijms26031196. Int J Mol Sci. 2025. PMID: 39940962 Free PMC article.
-
Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis.Breast Cancer Res. 2009;11(5):R68. doi: 10.1186/bcr2360. Breast Cancer Res. 2009. PMID: 19740433 Free PMC article.
-
Adhesion, metastasis, and inhibition of cancer cells: a comprehensive review.Mol Biol Rep. 2024 Jan 22;51(1):165. doi: 10.1007/s11033-023-08920-5. Mol Biol Rep. 2024. PMID: 38252369 Free PMC article. Review.
-
TGF-β signaling in lymphatic vascular vessel formation and maintenance.Front Physiol. 2022 Dec 15;13:1081376. doi: 10.3389/fphys.2022.1081376. eCollection 2022. Front Physiol. 2022. PMID: 36589453 Free PMC article. Review.
Cited by
-
β3 integrin expression is required for invadopodia-mediated ECM degradation in lung carcinoma cells.PLoS One. 2017 Aug 2;12(8):e0181579. doi: 10.1371/journal.pone.0181579. eCollection 2017. PLoS One. 2017. PMID: 28767724 Free PMC article.
-
Targeting TGF-β Signaling for Therapeutic Gain.Cold Spring Harb Perspect Biol. 2017 Oct 3;9(10):a022301. doi: 10.1101/cshperspect.a022301. Cold Spring Harb Perspect Biol. 2017. PMID: 28246179 Free PMC article. Review.
-
Advances in lymphatic metastasis of non-small cell lung cancer.Cell Commun Signal. 2024 Apr 2;22(1):201. doi: 10.1186/s12964-024-01574-1. Cell Commun Signal. 2024. PMID: 38566083 Free PMC article. Review.
-
Long non-coding RNA MFSD4A-AS1 promotes lymphangiogenesis and lymphatic metastasis of papillary thyroid cancer.Endocr Relat Cancer. 2023 Feb 8;30(3):e220221. doi: 10.1530/ERC-22-0221. Print 2023 Mar 1. Endocr Relat Cancer. 2023. PMID: 36606578 Free PMC article.
-
Integrin α5 promotes migration and invasion through the FAK/STAT3/AKT signaling pathway in icotinib-resistant non-small cell lung cancer cells.Oncol Lett. 2021 Jul;22(1):556. doi: 10.3892/ol.2021.12817. Epub 2021 May 24. Oncol Lett. 2021. PMID: 34084223 Free PMC article.
References
-
- Malkoski S, Haeger S, Cleaver T, Rodriguez K, Li H, Lu S, Feser W, Barón A, Merrick D, Lighthall J, Ijichi H, Franklin W, Wang X. Loss of transforming growth factor beta type II receptor increases aggressive tumor behavior and reduces survival in lung adenocarcinoma and squamous cell carcinoma. Clin Cancer Res. 2012;18(8):2173–2183. doi: 10.1158/1078-0432.CCR-11-2557. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

