Cost-effectiveness of enzyme replacement therapy with alglucosidase alfa in classic-infantile patients with Pompe disease
- PMID: 24884717
- PMCID: PMC4038090
- DOI: 10.1186/1750-1172-9-75
Cost-effectiveness of enzyme replacement therapy with alglucosidase alfa in classic-infantile patients with Pompe disease
Abstract
Background: Infantile Pompe disease is a rare metabolic disease. Patients generally do not survive the first year of life. Enzyme replacement therapy (ERT) has proven to have substantial effects on survival in infantile Pompe disease. However, the costs of therapy are very high. In this paper, we assess the cost-effectiveness of enzyme replacement therapy in infantile Pompe disease.
Methods: A patient simulation model was used to compare costs and effects of ERT with costs of effects of supportive therapy (ST). The model was filled with data on survival, quality of life and costs. For both arms of the model, data on survival were obtained from international literature. In addition, survival as observed among 20 classic-infantile Dutch patients, who all received ERT, was used. Quality of life was measured using the EQ-5D and assumed to be the same in both treatment groups. Costs included the costs of ERT (which depend on a child's weight), infusions, costs of other health care utilization, and informal care. A lifetime time horizon was used, with 6-month time cycles.
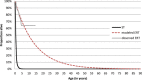
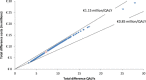
Results: Life expectancy was significantly longer in the ERT group than in the ST group. On average, ST receiving patients were modelled not to survive the first half year of life; whereas the life expectancy in the ERT patients was modelled to be almost 14 years. Lifetime incremental QALYs were 6.8. Incremental costs were estimated to be € 7.0 million, which primarily consisted of treatment costs (95%). The incremental costs per QALY were estimated to be € 1.0 million (range sensitivity analyses: € 0.3 million - € 1.3 million). The incremental cost per life year gained was estimated to be € 0.5 million.
Conclusions: The incremental costs per QALY ratio is far above the conventional threshold values. Results from univariate and probabilistic sensitivity analyses showed the robustness of the results.
Figures
Similar articles
-
Cost-effectiveness of enzyme replacement therapy with alglucosidase alfa in adult patients with Pompe disease.Orphanet J Rare Dis. 2017 Dec 13;12(1):179. doi: 10.1186/s13023-017-0731-0. Orphanet J Rare Dis. 2017. PMID: 29237491 Free PMC article.
-
The cost-effectiveness of enzyme replacement therapy (ERT) for the infantile form of Pompe disease: comparing a high-income country's approach (England) to that of a middle-income one (Colombia).Rev Salud Publica (Bogota). 2012 Jan-Feb;14(1):143-55. doi: 10.1590/s0124-00642012000100012. Rev Salud Publica (Bogota). 2012. PMID: 23250322
-
Effect of alglucosidase alfa dosage on survival and walking ability in patients with classic infantile Pompe disease: a multicentre observational cohort study from the European Pompe Consortium.Lancet Child Adolesc Health. 2022 Jan;6(1):28-37. doi: 10.1016/S2352-4642(21)00308-4. Epub 2021 Nov 22. Lancet Child Adolesc Health. 2022. PMID: 34822769
-
Improving the treatment of Pompe disease with enzyme replacement therapy: current strategies and clinical evidence.Expert Opin Pharmacother. 2025 May;26(7):835-848. doi: 10.1080/14656566.2025.2491508. Epub 2025 Apr 16. Expert Opin Pharmacother. 2025. PMID: 40237692 Review.
-
Clinical insight meets scientific innovation to develop a next generation ERT for Pompe disease.Mol Genet Metab. 2024 Sep-Oct;143(1-2):108559. doi: 10.1016/j.ymgme.2024.108559. Epub 2024 Aug 3. Mol Genet Metab. 2024. PMID: 39154400 Review.
Cited by
-
State of rare disease management in Southeast Asia.Orphanet J Rare Dis. 2016 Aug 2;11(1):107. doi: 10.1186/s13023-016-0460-9. Orphanet J Rare Dis. 2016. PMID: 27484654 Free PMC article.
-
Cost-Effectiveness of Ventricular Assist Device Destination Therapy for Advanced Heart Failure in Duchenne Muscular Dystrophy.Pediatr Cardiol. 2018 Aug;39(6):1242-1248. doi: 10.1007/s00246-018-1889-5. Epub 2018 May 17. Pediatr Cardiol. 2018. PMID: 29774392
-
Intracranial arterial abnormalities in patients with late onset Pompe disease (LOPD).J Inherit Metab Dis. 2016 May;39(3):391-398. doi: 10.1007/s10545-015-9913-x. Epub 2016 Feb 1. J Inherit Metab Dis. 2016. PMID: 26830551
-
Therapeutics in paediatric genetic diseases: Current and future landscape.Singapore Med J. 2023 Jan;64(1):7-16. doi: 10.4103/singaporemedj.SMJ-2021-376. Singapore Med J. 2023. PMID: 36722512 Free PMC article. Review.
-
Induced pluripotent stem cell models of lysosomal storage disorders.Dis Model Mech. 2017 Jun 1;10(6):691-704. doi: 10.1242/dmm.029009. Dis Model Mech. 2017. PMID: 28592657 Free PMC article. Review.
References
-
- Drummond M, Evans B, LeLorier J, Karakiewicz P, Martin D, Tugwell P, MacLeod S. Evidence and values: requirements for public reimbursement of drugs for rare diseases–a case study in oncology. Can J Clin Pharmacol. 2009;16:e273–e281. - PubMed
-
- Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess Health Care. 2007;23:425–432. - PubMed
-
- Garau M, Mestre-Ferrandiz J. Access mechanisms for orphan drugs: a comparative study of selected European countries. Off Health Econ. 2009;52:1–30.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous