USP18 is crucial for IFN-γ-mediated inhibition of B16 melanoma tumorigenesis and antitumor immunity
- PMID: 24884733
- PMCID: PMC4057584
- DOI: 10.1186/1476-4598-13-132
USP18 is crucial for IFN-γ-mediated inhibition of B16 melanoma tumorigenesis and antitumor immunity
Abstract
Background: Interferon (IFN)-γ-mediated immune response plays an important role in tumor immunosurveillance. However, the regulation of IFN-γ-mediated tumorigenesis and immune response remains elusive. USP18, an interferon stimulating response element, regulates IFN-α-mediated signaling in anti-viral immune response, but its role in IFN-γ-mediated tumorigenesis and anti-tumor immune response is unknown.
Method: In this study, USP18 in tumorigenesis and anti-tumor immune response was comprehensively appraised in vivo by overexpression or downregulation its expression in murine B16 melanoma tumor model in immunocompetent and immunodeficient mice.
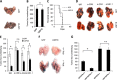
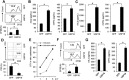
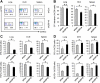
Results: Ectopic expression or downregulation of USP18 in B16 melanoma tumor cells inhibited or promoted tumorigenesis, respectively, in immunocompetent mice. USP18 expression in B16 melanoma tumor cells regulated IFN-γ-mediated immunoediting, including upregulating MHC class-I expression, reducing tumor cell-mediated inhibition of T cell proliferation and activation, and suppressing PD-1 expression in CD4+ and CD8+ T cells in tumor-bearing mice. USP18 expression in B16 melanoma tumor cells also enhanced CTL activity during adoptive immunotherapy by prolonging the persistence and enhancing the activity of adoptively transferred CTLs and by reducing CTL exhaustion in the tumor microenvironment. Mechanistic studies demonstrated that USP18 suppressed tumor cell-mediated immune inhibition by activating T cells, inhibiting T-cell exhaustion, and reducing dendritic cell tolerance, thus sensitizing tumor cells to immunosurveillance and immunotherapy.
Conclusion: These findings suggest that stimulating USP18 is a feasible approach to induce B16 melanoma specific immune response.
Figures




Similar articles
-
Acceleration of pancreatic tumorigenesis under immunosuppressive microenvironment induced by Reg3g overexpression.Cell Death Dis. 2017 Sep 7;8(9):e3033. doi: 10.1038/cddis.2017.424. Cell Death Dis. 2017. PMID: 28880262 Free PMC article.
-
Intratumoral injection of interferon-gamma gene-modified dendritic cells elicits potent antitumor effects: effective induction of tumor-specific CD8+ CTL response.J Cancer Res Clin Oncol. 2005 Jul;131(7):468-78. doi: 10.1007/s00432-004-0651-y. Epub 2005 Feb 12. J Cancer Res Clin Oncol. 2005. PMID: 15711825 Free PMC article.
-
Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma.J Immunol. 2003 Dec 1;171(11):5940-7. doi: 10.4049/jimmunol.171.11.5940. J Immunol. 2003. PMID: 14634105
-
The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion.Int J Mol Sci. 2017 Dec 28;19(1):89. doi: 10.3390/ijms19010089. Int J Mol Sci. 2017. PMID: 29283429 Free PMC article. Review.
-
The importance of type I interferon in orchestrating the cytotoxic T-cell response to cancer.Immunol Lett. 2024 Dec;270:106938. doi: 10.1016/j.imlet.2024.106938. Epub 2024 Oct 28. Immunol Lett. 2024. PMID: 39490629 Review.
Cited by
-
Deubiquitinases as novel therapeutic targets for diseases.MedComm (2020). 2024 Dec 13;5(12):e70036. doi: 10.1002/mco2.70036. eCollection 2024 Dec. MedComm (2020). 2024. PMID: 39678489 Free PMC article. Review.
-
Type I interferon regulation by USP18 is a key vulnerability in cancer.iScience. 2024 Mar 27;27(4):109593. doi: 10.1016/j.isci.2024.109593. eCollection 2024 Apr 19. iScience. 2024. PMID: 38632987 Free PMC article.
-
Downregulation of USP18 reduces tumor-infiltrating activated dendritic cells in extranodal diffuse large B cell lymphoma patients.Aging (Albany NY). 2021 May 17;13(10):14131-14158. doi: 10.18632/aging.203030. Epub 2021 May 17. Aging (Albany NY). 2021. PMID: 34001679 Free PMC article.
-
USP18-deficiency in cervical carcinoma is crucial for the malignant behavior of tumor cells in an ERK signal-dependent manner.Oncol Lett. 2021 May;21(5):421. doi: 10.3892/ol.2021.12682. Epub 2021 Mar 29. Oncol Lett. 2021. PMID: 33850562 Free PMC article.
-
Abnormal expression of circulating and tumor-infiltrating carcinoembryonic antigen-related cell adhesion molecule 1 in patients with glioma.Oncol Lett. 2018 Mar;15(3):3496-3503. doi: 10.3892/ol.2018.7786. Epub 2018 Jan 12. Oncol Lett. 2018. PMID: 29467871 Free PMC article.
References
-
- van Hall T, Wolpert EZ, van Veelen P, Laban S, van der Veer M, Roseboom M, Bres S, Grufman P, de Ru A, Meiring H, de Jong A, Franken K, Teixeira A, Valentijn R, Drijfhout JW, Koning F, Camps M, Ossendorp F, Karre K, Ljunggren HG, Melief CJ, Offringa R. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12:417–424. doi: 10.1038/nm1381. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

