Critical role of cellular cholesterol in bovine rotavirus infection
- PMID: 24884772
- PMCID: PMC4053397
- DOI: 10.1186/1743-422X-11-98
Critical role of cellular cholesterol in bovine rotavirus infection
Abstract
Background: Bovine rotavirus (BRV) is a non-enveloped dsRNA virus that cause neonatal calf diarrhea. Lipid rafts are cholesterol-enrich membrane mircodomains that play a vital role in many cellular processes. In this study, the effect of cellular cholesterol depletion on infection of MA-104 cells with bovine rotavirus was investigated.
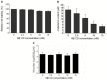
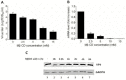
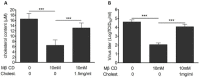
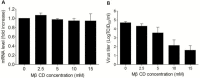
Results: We demonstrated that cholesterol depletion of the plasma membrane by MβCD had no effect on BRV binding to cells but significantly impaired BRV entry in a dose-dependent manner and the effect was partially reversed by addition of exogenous cholesterol, suggesting the reduction of BRV infection by MβCD was specifically due to cholesterol depletion. Cholesterol depletion after virus entry did not reduce BRV replication, whereas affected virus assembly.
Conclusions: Taken together, our results demonstrate that cell membrane cholesterol is essential to BRV infectivity.
Figures
Similar articles
-
Cholesterol of lipid rafts is a key determinant for entry and post-entry control of porcine rotavirus infection.BMC Vet Res. 2018 Feb 12;14(1):45. doi: 10.1186/s12917-018-1366-7. BMC Vet Res. 2018. PMID: 29433482 Free PMC article.
-
Cholesterol dependence of pseudorabies herpesvirus entry.Curr Microbiol. 2011 Jan;62(1):261-6. doi: 10.1007/s00284-010-9700-8. Epub 2010 Jul 13. Curr Microbiol. 2011. PMID: 20625735 Free PMC article.
-
Cholesterol-rich lipid rafts both in cellular and viral membrane are critical for caprine parainfluenza virus type3 entry and infection in host cells.Vet Microbiol. 2020 Sep;248:108794. doi: 10.1016/j.vetmic.2020.108794. Epub 2020 Jul 23. Vet Microbiol. 2020. PMID: 32827922
-
Prevalence of bovine rotavirus among Bovidae in China during 1984-2021: A systematic review and meta-analysis.Microb Pathog. 2022 Aug;169:105661. doi: 10.1016/j.micpath.2022.105661. Epub 2022 Jul 8. Microb Pathog. 2022. PMID: 35817280
-
Structural Insights into Rotavirus Entry.Adv Exp Med Biol. 2019;1215:45-68. doi: 10.1007/978-3-030-14741-9_3. Adv Exp Med Biol. 2019. PMID: 31317495 Review.
Cited by
-
In Vitro Antiviral Activity of Germacrone Against Porcine Reproductive and Respiratory Syndrome Virus.Curr Microbiol. 2016 Sep;73(3):317-323. doi: 10.1007/s00284-016-1042-8. Epub 2016 May 13. Curr Microbiol. 2016. PMID: 27178541
-
Restriction fragment length polymorphism analysis of rotavirus VP7-encoding gene from humans and animals of Northeast India: a relative study of Indian and global isolates.Epidemiol Infect. 2015 Sep;143(12):2503-11. doi: 10.1017/S0950268814003343. Epub 2015 Jan 9. Epidemiol Infect. 2015. PMID: 25573161 Free PMC article.
-
Treading a HOSTile path: Mapping the dynamic landscape of host cell-rotavirus interactions to explore novel host-directed curative dimensions.Virulence. 2021 Dec;12(1):1022-1062. doi: 10.1080/21505594.2021.1903198. Virulence. 2021. PMID: 33818275 Free PMC article. Review.
-
Host Cell Response to Rotavirus Infection with Emphasis on Virus-Glycan Interactions, Cholesterol Metabolism, and Innate Immunity.Viruses. 2023 Jun 21;15(7):1406. doi: 10.3390/v15071406. Viruses. 2023. PMID: 37515094 Free PMC article.
-
First detection and genomic characteristics of bovine torovirus in dairy calves in China.Arch Virol. 2020 Jul;165(7):1577-1583. doi: 10.1007/s00705-020-04657-9. Epub 2020 May 9. Arch Virol. 2020. PMID: 32388597 Free PMC article.
References
-
- Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gómara M, Johne R, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Parreño V, Rahman M, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Patton JT, Desselberger U, Van Ranst M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG) Arch Virol. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

