Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits
- PMID: 24884783
- PMCID: PMC4067080
- DOI: 10.1186/1742-4690-11-41
Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits
Abstract
Background: The trimeric envelope glycoproteins (Env) on the surface of HIV-1 virions are the targets for neutralizing antibodies (NAbs). No candidate HIV-1 immunogen has yet induced potent, broadly active NAbs (bNAbs). Part of the explanation may be that previously tested Env proteins inadequately mimic the functional, native Env complex. Trimerization and the proteolytic processing of Env precursors into gp120 and gp41 profoundly alter antigenicity, but soluble cleaved trimers are too unstable to serve as immunogens. By introducing stabilizing mutations (SOSIP), we constructed soluble, cleaved Env trimers derived from the HIV-1 subtype A isolate BG505 that resemble native Env spikes on virions both structurally and antigenically.
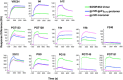
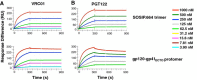
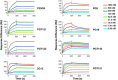
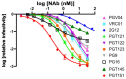
Results: We used surface plasmon resonance (SPR) to quantify antibody binding to different forms of BG505 Env: the proteolytically cleaved SOSIP.664 trimers, cleaved gp120-gp41ECTO protomers, and gp120 monomers. Non-NAbs to the CD4-binding site bound only marginally to the trimers but equally well to gp120-gp41ECTO protomers and gp120 monomers, whereas the bNAb VRC01, directed to the CD4bs, bound to all three forms. In contrast, bNAbs to V1V2 glycan-dependent epitopes bound preferentially (PG9 and PG16) or exclusively (PGT145) to trimers. We also explored the antigenic consequences of three different features of SOSIP.664 gp140 trimers: the engineered inter-subunit disulfide bond, the trimer-stabilizing I559P change in gp41ECTO, and proteolytic cleavage at the gp120-gp41ECTO junction. Each of these three features incrementally promoted native-like trimer antigenicity. We compared Fab and IgG versions of bNAbs and validated a bivalent model of IgG binding. The NAbs showed widely divergent binding kinetics and degrees of binding to native-like BG505 SOSIP.664. High off-rate constants and low stoichiometric estimates of NAb binding were associated with large amounts of residual infectivity after NAb neutralization of the corresponding BG505.T332N pseudovirus.
Conclusions: The antigenicity and structural integrity of cleaved BG505 SOSIP.664 trimers render these proteins good mimics of functional Env spikes on virions. In contrast, uncleaved gp140s antigenically resemble individual gp120-gp41ECTO protomers and gp120 monomers, but not native trimers. Although NAb binding to functional trimers may thus be both necessary and sufficient for neutralization, the kinetics and stoichiometry of the interaction influence the neutralizing efficacy of individual NAbs.
Figures

Similar articles
-
A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies.PLoS Pathog. 2013 Sep;9(9):e1003618. doi: 10.1371/journal.ppat.1003618. Epub 2013 Sep 19. PLoS Pathog. 2013. PMID: 24068931 Free PMC article.
-
Single-Chain Soluble BG505.SOSIP gp140 Trimers as Structural and Antigenic Mimics of Mature Closed HIV-1 Env.J Virol. 2015 May;89(10):5318-29. doi: 10.1128/JVI.03451-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740988 Free PMC article.
-
Immunogenicity of a Prefusion HIV-1 Envelope Trimer in Complex with a Quaternary-Structure-Specific Antibody.J Virol. 2015 Dec 30;90(6):2740-55. doi: 10.1128/JVI.02380-15. J Virol. 2015. PMID: 26719262 Free PMC article.
-
Native-like Env trimers as a platform for HIV-1 vaccine design.Immunol Rev. 2017 Jan;275(1):161-182. doi: 10.1111/imr.12481. Immunol Rev. 2017. PMID: 28133806 Free PMC article. Review.
-
Immunogen design to focus the B-cell repertoire.Curr Opin HIV AIDS. 2014 May;9(3):217-23. doi: 10.1097/COH.0000000000000054. Curr Opin HIV AIDS. 2014. PMID: 24675068 Review.
Cited by
-
Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives.Adv Biol. 2014;2014:157895. doi: 10.1155/2014/157895. Epub 2014 Sep 9. Adv Biol. 2014. PMID: 27099867 Free PMC article.
-
Star nanoparticles delivering HIV-1 peptide minimal immunogens elicit near-native envelope antibody responses in nonhuman primates.PLoS Biol. 2019 Jun 17;17(6):e3000328. doi: 10.1371/journal.pbio.3000328. eCollection 2019 Jun. PLoS Biol. 2019. PMID: 31206510 Free PMC article.
-
Assessment of Antibody Interference of Enfuvirtide (T20) Function Shows Assay Dependent Variability.Curr HIV Res. 2018;16(6):404-415. doi: 10.2174/1570162X17666190228154850. Curr HIV Res. 2018. PMID: 30836922 Free PMC article.
-
Germline-targeting HIV vaccination induces neutralizing antibodies to the CD4 binding site.Sci Immunol. 2024 Aug 30;9(98):eadk9550. doi: 10.1126/sciimmunol.adk9550. Epub 2024 Aug 30. Sci Immunol. 2024. PMID: 39213338 Free PMC article.
-
The HIV-1 envelope glycoprotein: structure, function and interactions with neutralizing antibodies.Nat Rev Microbiol. 2025 Jul 23. doi: 10.1038/s41579-025-01206-6. Online ahead of print. Nat Rev Microbiol. 2025. PMID: 40702326 Review.
References
-
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

