Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences
- PMID: 24884833
- PMCID: PMC4038707
- DOI: 10.1186/1471-2202-15-65
Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences
Abstract
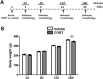
Background: Glucocorticoid released by stressful stimuli elicits various stress responses. Acute treatment with a single dose of corticosterone (CORT; predominant glucocorticoid of rats) alone has previously been shown to trigger anxiety behavior and robust dendritic hypertrophy of neurons in the basolateral amygdala (BLA). Neurons in the medial prefrontal cortex (mPFC) are also known to be highly sensitive to stress and regulate anxiety-like behaviors. Nevertheless, we know less about acute CORT-induced structural changes of other brain regions and their behavioral outcomes. In addition, the temporal profile of acute CORT effects remains to be examined. The current study investigates time course changes of dendritic architectures in the stress vulnerable brain areas, the BLA and mPFC, and their behavioral consequences after acute treatment with a single dose of CORT.
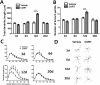
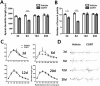
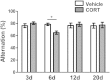
Results: Acute CORT treatment produced delayed onset of dendritic remodeling in the opposite direction in the BLA and mPFC with different time courses. Acute CORT induced dendritic hypertrophy of BLA spiny neurons, which was paralleled by heightened anxiety, both peaked 12 days after the treatment. Meanwhile, CORT-induced dendritic atrophy of mPFC pyramidal neurons peaked on day 6, concomitantly with impaired working memory. Both changed dendritic morphologies and altered behavioral outcomes were fully recovered.
Conclusion: Our results suggest that stress-induced heightened anxiety appears to be a functional consequence of dendritic remodeling of BLA neurons but not that of mPFC. Instead, stress-induced dendritic atrophy of mPFC neurons is relevant to working memory deficit. Therefore, structural changes in the BLA and the mPFC might be specifically associated with distinct behavioral symptoms observed in stress-related mental disorders. Remarkably, stress-induced dendritic remodeling in the BLA as well as mPFC is readily reversible. The related behavioral outcomes also follow the similar time course in a reversible manner. Therefore, further studies on the cellular mechanism for the plasticity of dendrites architecture might provide new insight into the etiological factors for stress-related mental illness such as posttraumatic stress disorder (PTSD).
Figures

Similar articles
-
Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy.Proc Natl Acad Sci U S A. 2008 Apr 8;105(14):5573-8. doi: 10.1073/pnas.0705615105. Epub 2008 Apr 7. Proc Natl Acad Sci U S A. 2008. PMID: 18391224 Free PMC article.
-
Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat.J Chem Neuroanat. 2009 Dec;38(4):266-72. doi: 10.1016/j.jchemneu.2009.05.009. Epub 2009 Jun 6. J Chem Neuroanat. 2009. PMID: 19505571
-
Lavender essential oil ameliorates depression-like behavior and increases neurogenesis and dendritic complexity in rats.Neurosci Lett. 2019 May 14;701:180-192. doi: 10.1016/j.neulet.2019.02.042. Epub 2019 Feb 28. Neurosci Lett. 2019. PMID: 30825591
-
Stress, anxiety, and dendritic spines: what are the connections?Neuroscience. 2013 Oct 22;251:108-19. doi: 10.1016/j.neuroscience.2012.04.021. Epub 2012 Apr 20. Neuroscience. 2013. PMID: 22522470 Review.
-
Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress.Neuropharmacology. 2012 Jan;62(1):3-12. doi: 10.1016/j.neuropharm.2011.07.014. Epub 2011 Jul 27. Neuropharmacology. 2012. PMID: 21807003 Free PMC article. Review.
Cited by
-
Sex-Specific Role for SLIT1 in Regulating Stress Susceptibility.Biol Psychiatry. 2022 Jan 1;91(1):81-91. doi: 10.1016/j.biopsych.2021.01.019. Epub 2021 Feb 27. Biol Psychiatry. 2022. PMID: 33896623 Free PMC article.
-
Early Life Stress Effects on Glucocorticoid-BDNF Interplay in the Hippocampus.Front Mol Neurosci. 2015 Nov 16;8:68. doi: 10.3389/fnmol.2015.00068. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26635521 Free PMC article. Review.
-
Changed Synaptic Plasticity in Neural Circuits of Depressive-Like and Escitalopram-Treated Rats.Int J Neuropsychopharmacol. 2015 Apr 21;18(10):pyv046. doi: 10.1093/ijnp/pyv046. Int J Neuropsychopharmacol. 2015. PMID: 25899067 Free PMC article.
-
miR-132/212 is induced by stress and its dysregulation triggers anxiety-related behavior.Neuropharmacology. 2019 Jan;144:256-270. doi: 10.1016/j.neuropharm.2018.10.020. Epub 2018 Oct 18. Neuropharmacology. 2019. PMID: 30342060 Free PMC article.
-
Impact of stress on inhibitory neuronal circuits, our tribute to Bruce McEwen.Neurobiol Stress. 2022 May 13;19:100460. doi: 10.1016/j.ynstr.2022.100460. eCollection 2022 Jul. Neurobiol Stress. 2022. PMID: 35734023 Free PMC article.
References
-
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;15:112–119. doi: 10.1016/j.pnpbp.2012.05.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

