The Netrin-1 receptor DCC is a regulator of maladaptive responses to chronic morphine administration
- PMID: 24884839
- PMCID: PMC4038717
- DOI: 10.1186/1471-2164-15-345
The Netrin-1 receptor DCC is a regulator of maladaptive responses to chronic morphine administration
Abstract
Background: Opioids are the cornerstone of treatment for moderate to severe pain, but chronic use leads to maladaptations that include: tolerance, dependence and opioid-induced hyperalgesia (OIH). These responses limit the utility of opioids, as well as our ability to control chronic pain. Despite decades of research, we have no therapies or proven strategies to overcome this problem. However, murine haplotype based computational genetic mapping and a SNP data base generated from analysis of whole-genome sequence data (whole-genome HBCGM), provides a hypothesis-free method for discovering novel genes affecting opioid maladaptive responses.
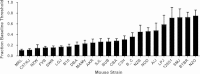
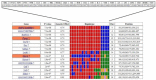
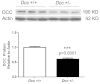
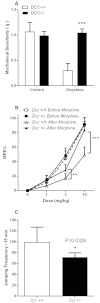
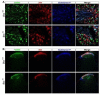
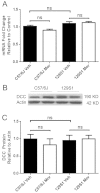
Results: Whole genome-HBCGM was used to analyze phenotypic data on morphine-induced tolerance, dependence and hyperalgesia obtained from 23 inbred strains. The robustness of the genetic mapping results was analyzed using strain subsets. In addition, the results of analyzing all of the opioid-related traits together were examined. To characterize the functional role of the leading candidate gene, we analyzed transgenic animals, mRNA and protein expression in behaviorally divergent mouse strains, and immunohistochemistry in spinal cord tissue. Our mapping procedure identified the allelic pattern within the netrin-1 receptor gene (Dcc) as most robustly associated with OIH, and it was also strongly associated with the combination of the other maladaptive opioid traits analyzed. Adult mice heterozygous for the Dcc gene had significantly less tendency to develop OIH, become tolerant or show evidence of dependence after chronic exposure to morphine. The difference in opiate responses was shown not to be due to basal or morphine-stimulated differences in the level of Dcc expression in spinal cord tissue, and was not associated with nociceptive neurochemical or anatomical alterations in the spinal cord or dorsal root ganglia in adult animals.
Conclusions: Whole-genome HBCGM is a powerful tool for identifying genes affecting biomedical traits such as opioid maladaptations. We demonstrate that Dcc affects tolerance, dependence and OIH after chronic opioid exposure, though not through simple differences in expression in the adult spinal cord.
Figures
Similar articles
-
The multiple PDZ domain protein Mpdz/MUPP1 regulates opioid tolerance and opioid-induced hyperalgesia.BMC Genomics. 2016 Apr 29;17:313. doi: 10.1186/s12864-016-2634-1. BMC Genomics. 2016. PMID: 27129385 Free PMC article.
-
Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice.J Pain. 2013 Jan;14(1):36-47. doi: 10.1016/j.jpain.2012.10.005. J Pain. 2013. PMID: 23273833 Free PMC article.
-
Epigenetic regulation of spinal cord gene expression controls opioid-induced hyperalgesia.Mol Pain. 2014 Sep 12;10:59. doi: 10.1186/1744-8069-10-59. Mol Pain. 2014. PMID: 25217253 Free PMC article.
-
Opioid-induced hyperalgesia: Cellular and molecular mechanisms.Neuroscience. 2016 Dec 3;338:160-182. doi: 10.1016/j.neuroscience.2016.06.029. Epub 2016 Jun 23. Neuroscience. 2016. PMID: 27346146 Review.
-
Knowing the Enemy Is Halfway towards Victory: A Scoping Review on Opioid-Induced Hyperalgesia.J Clin Med. 2022 Oct 19;11(20):6161. doi: 10.3390/jcm11206161. J Clin Med. 2022. PMID: 36294488 Free PMC article.
Cited by
-
Skeletal interoception in bone homeostasis and pain.Cell Metab. 2022 Dec 6;34(12):1914-1931. doi: 10.1016/j.cmet.2022.09.025. Epub 2022 Oct 17. Cell Metab. 2022. PMID: 36257317 Free PMC article. Review.
-
A systematic review of genome-wide association studies for pain, nociception, neuropathy, and pain treatment responses.Pain. 2023 Sep 1;164(9):1891-1911. doi: 10.1097/j.pain.0000000000002910. Epub 2023 May 5. Pain. 2023. PMID: 37144689 Free PMC article.
-
A Population-Based Study of Four Genes Associated with Heroin Addiction in Han Chinese.PLoS One. 2016 Sep 27;11(9):e0163668. doi: 10.1371/journal.pone.0163668. eCollection 2016. PLoS One. 2016. PMID: 27676367 Free PMC article.
-
Neuron Navigator 1 (Nav1) regulates the response to cocaine in mice.Commun Biol. 2023 Oct 18;6(1):1053. doi: 10.1038/s42003-023-05430-9. Commun Biol. 2023. PMID: 37853211 Free PMC article.
-
Preconception opioids interact with mouse strain to alter morphine withdrawal in the next generation.Psychopharmacology (Berl). 2024 Jul;241(7):1435-1446. doi: 10.1007/s00213-024-06574-0. Epub 2024 Mar 19. Psychopharmacology (Berl). 2024. PMID: 38503843
References
-
- Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

