A model-based analysis: what potential could there be for a S. aureus vaccine in a hospital setting on top of other preventative measures?
- PMID: 24884845
- PMCID: PMC4046499
- DOI: 10.1186/1471-2334-14-291
A model-based analysis: what potential could there be for a S. aureus vaccine in a hospital setting on top of other preventative measures?
Abstract
Background: Over the past decade, there has been sustained interest and efforts to develop a S. aureus vaccine. There is a need to better evaluate the potential public health impact of S. aureus vaccination, particularly given that preventative measures exist to reduce infection. To our knowledge, there is no previous work to assess the potential of a S. aureus vaccine to yield additional MRSA infection reduction in a hospital setting, on top of other preventative measures that already proved efficient.
Methods: The main objectives were to propose a versatile simulation framework for assessing potential added benefits of a hypothetical S. Aureus vaccine in conjunction with other preventative measures, and to illustrate possibilities in a given hospital setting. To this end, we employed a recently published dynamic transmission modelling framework that we further adapted and expanded to include a hypothetical S. aureus vaccination component in order to estimate potential benefits of vaccinating patients prior to hospital admission.
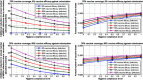
Results: Model-based projections indicate that even with other hygiene prevention measures in place, vaccination of patients prior to hospital admission has the potential to provide additional reduction of MRSA infection. Vaccine coverage and vaccine efficacy are key factors that would ultimately impact the magnitude of this reduction. For example, in an average case scenario with 50% decolonization, 50% screening and 50% hygiene compliance level in place, S. aureus vaccination with 25% vaccine coverage, 75% vaccine efficacy against infection, and 0% vaccine efficacy against colonization, may lead to 12% model-projected additional reduction in MRSA infection prevalence due to vaccination, while this reduction could reach 37% for vaccination with 75% vaccine coverage and 75% vaccine efficacy against infection in the same average case scenario.
Conclusions: S. aureus vaccination could potentially provide additional reduction of MRSA infection in a hospital setting, on top of reductions from hygiene prevention measures. The magnitude of such additional reductions can vary significantly depending on the level of hygiene prevention measures in place, as well as key vaccine factors such as coverage and efficacy. Identifying appropriate combinations of preventative measures may lead to optimal strategies to effectively reduce MRSA infection in hospitals.
Figures




Similar articles
-
Evaluating the potential public health impact of a Staphylococcus aureus vaccine through use of population-based surveillance for invasive methicillin-resistant S. aureus disease in the United States.Vaccine. 2009 Aug 13;27(37):5061-8. doi: 10.1016/j.vaccine.2009.06.055. Epub 2009 Jul 2. Vaccine. 2009. PMID: 19576943
-
Development and evaluation of vaccine against Staphylococcus aureus recovered from naturally occurring mastitis in she-camels.Microb Pathog. 2018 Apr;117:341-347. doi: 10.1016/j.micpath.2018.03.003. Epub 2018 Mar 3. Microb Pathog. 2018. PMID: 29510207
-
Whole-genome epidemiology, characterisation, and phylogenetic reconstruction of Staphylococcus aureus strains in a paediatric hospital.Genome Med. 2018 Nov 13;10(1):82. doi: 10.1186/s13073-018-0593-7. Genome Med. 2018. PMID: 30424799 Free PMC article.
-
Screening and decolonization: does methicillin-susceptible Staphylococcus aureus hold lessons for methicillin-resistant S. aureus?Clin Infect Dis. 2010 Sep 1;51(5):585-90. doi: 10.1086/655695. Clin Infect Dis. 2010. PMID: 20662715 Review.
-
Control and prevention of MRSA infections.Methods Mol Biol. 2007;391:209-25. doi: 10.1007/978-1-59745-468-1_16. Methods Mol Biol. 2007. PMID: 18025680 Review.
Cited by
-
PIA and rSesC Mixture Arisen Antibodies Could Inhibit the Biofilm-Formation in Staphylococcus aureus.Rep Biochem Mol Biol. 2021 Apr;10(1):1-12. doi: 10.52547/rbmb.10.1.1. Rep Biochem Mol Biol. 2021. PMID: 34277863 Free PMC article.
-
Using contact network dynamics to implement efficient interventions against pathogen spread in hospital settings: A modelling study.PLoS Med. 2024 Jul 30;21(7):e1004433. doi: 10.1371/journal.pmed.1004433. eCollection 2024 Jul. PLoS Med. 2024. PMID: 39078828 Free PMC article.
-
Staphylococcus aureus Putative Vaccines Based on the Virulence Factors: A Mini-Review.Front Microbiol. 2021 Sep 3;12:704247. doi: 10.3389/fmicb.2021.704247. eCollection 2021. Front Microbiol. 2021. PMID: 34539603 Free PMC article. Review.
-
Improving mathematical modeling of interventions to prevent healthcare-associated infections by interrupting transmission or pathogens: How common modeling assumptions about colonized individuals impact intervention effectiveness estimates.PLoS One. 2022 Feb 28;17(2):e0264344. doi: 10.1371/journal.pone.0264344. eCollection 2022. PLoS One. 2022. PMID: 35226689 Free PMC article.
-
Implications of asymptomatic carriers for infectious disease transmission and control.R Soc Open Sci. 2018 Feb 14;5(2):172341. doi: 10.1098/rsos.172341. eCollection 2018 Feb. R Soc Open Sci. 2018. PMID: 29515909 Free PMC article.
References
-
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;14:1436–1444. doi: 10.1056/NEJMoa043252. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

