Membrane-bound IL-12 and IL-23 serve as potent mucosal adjuvants when co-presented on whole inactivated influenza vaccines
- PMID: 24884849
- PMCID: PMC4036309
- DOI: 10.1186/1743-422X-11-78
Membrane-bound IL-12 and IL-23 serve as potent mucosal adjuvants when co-presented on whole inactivated influenza vaccines
Abstract
Background: Potent and safe adjuvants are needed to improve the efficacy of parenteral and mucosal vaccines. Cytokines, chemokines and growth factors have all proven to be effective immunomodulatory adjuvants when administered with a variety of antigens. We have previously evaluated the efficacy of membrane-anchored interleukins (IL) such as IL-2 and IL-4 co-presented as Cytokine-bearing Influenza Vaccines (CYT-IVACs) using a mouse model of influenza challenge.
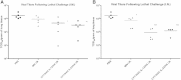
Findings: Here, we describe studies evaluating the parenteral and mucosal adjuvanticity of membrane-bound IL-12 and IL-23 CYT-IVACs in young adult mice. Mucosal immunization using IL-12 and IL-23 bearing whole influenza virus vaccine (WIV) was more effective at eliciting virus-specific nasal IgA and reducing viral lung burden following challenge compared to control WIV vaccinated animals. Both IL-12 and IL-23 bearing WIV elicited the highest anti-viral IgA levels in serum and nasal washes.
Conclusions: This study highlights for the first time the mucosal adjuvant potential of IL-12 and IL-23 CYT-IVAC formulations in eliciting mucosal immune responses and reducing viral lung burden. The co-presentation of immunomodulators in direct context with viral antigen in whole inactivated viral vaccines may provide a means to significantly lower the dose of vaccine required for protection.
Figures



Similar articles
-
Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection.Vaccine. 2012 Jan 5;30(2):466-74. doi: 10.1016/j.vaccine.2011.10.058. Epub 2011 Oct 31. Vaccine. 2012. PMID: 22051136
-
Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration.Vaccine. 2012 Jul 6;30(32):4884-91. doi: 10.1016/j.vaccine.2012.04.032. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537989
-
An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice.J Virol. 2014 Aug;88(16):9182-96. doi: 10.1128/JVI.00327-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899195 Free PMC article.
-
Studies on the usefulness of intranasal inactivated influenza vaccines.Vaccine. 2010 Aug 31;28(38):6393-7. doi: 10.1016/j.vaccine.2010.05.019. Epub 2010 May 20. Vaccine. 2010. PMID: 20493820 Review.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
Cited by
-
Innovative Mucosal Vaccine Formulations Against Influenza A Virus Infections.Front Immunol. 2019 Jul 17;10:1605. doi: 10.3389/fimmu.2019.01605. eCollection 2019. Front Immunol. 2019. PMID: 31379823 Free PMC article. Review.
-
The potential of a universal influenza virus-like particle vaccine expressing a chimeric cytokine.Life Sci Alliance. 2022 Nov 7;6(1):e202201548. doi: 10.26508/lsa.202201548. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36344085 Free PMC article.
-
Alkyl polyglycoside, a highly promising adjuvant in intranasal split influenza vaccines.Hum Vaccin Immunother. 2017 Jun 3;13(6):1-9. doi: 10.1080/21645515.2016.1278098. Epub 2017 Jan 27. Hum Vaccin Immunother. 2017. PMID: 28129034 Free PMC article.
-
Interleukin 12-containing influenza virus-like-particle vaccine elevate its protective activity against heterotypic influenza virus infection.Heliyon. 2020 Aug 8;6(8):e04543. doi: 10.1016/j.heliyon.2020.e04543. eCollection 2020 Aug. Heliyon. 2020. PMID: 32802975 Free PMC article.
References
-
- Furuya Y. Return of inactivated whole-virus vaccine for superior efficacy. Immunol Cell Biol. 2011;90:571–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

