Clinician-led improvement in cancer care (CLICC)--testing a multifaceted implementation strategy to increase evidence-based prostate cancer care: phased randomised controlled trial--study protocol
- PMID: 24884877
- PMCID: PMC4048539
- DOI: 10.1186/1748-5908-9-64
Clinician-led improvement in cancer care (CLICC)--testing a multifaceted implementation strategy to increase evidence-based prostate cancer care: phased randomised controlled trial--study protocol
Abstract
Background: Clinical practice guidelines have been widely developed and disseminated with the aim of improving healthcare processes and patient outcomes but the uptake of evidence-based practice remains haphazard. There is a need to develop effective implementation methods to achieve large-scale adoption of proven innovations and recommended care. Clinical networks are increasingly being viewed as a vehicle through which evidence-based care can be embedded into healthcare systems using a collegial approach to agree on and implement a range of strategies within hospitals. In Australia, the provision of evidence-based care for men with prostate cancer has been identified as a high priority. Clinical audits have shown that fewer than 10% of patients in New South Wales (NSW) Australia at high risk of recurrence after radical prostatectomy receive guideline recommended radiation treatment following surgery. This trial will test a clinical network-based intervention to improve uptake of guideline recommended care for men with high-risk prostate cancer.
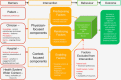
Methods/design: In Phase I, a phased randomised cluster trial will test a multifaceted intervention that harnesses the NSW Agency for Clinical Innovation (ACI) Urology Clinical Network to increase evidence-based care for men with high-risk prostate cancer following surgery. The intervention will be introduced in nine NSW hospitals over 10 months using a stepped wedge design. Outcome data (referral to radiation oncology for discussion of adjuvant radiotherapy in line with guideline recommended care or referral to a clinical trial of adjuvant versus salvage radiotherapy) will be collected through review of patient medical records. In Phase II, mixed methods will be used to identify mechanisms of provider and organisational change. Clinicians' knowledge and attitudes will be assessed through surveys. Process outcome measures will be assessed through document review. Semi-structured interviews will be conducted to elucidate mechanisms of change.
Discussion: The study will be one of the first randomised controlled trials to test the effectiveness of clinical networks to lead changes in clinical practice in hospitals treating patients with high-risk cancer. It will additionally provide direction regarding implementation strategies that can be effectively employed to encourage widespread adoption of clinical practice guidelines.
Trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12611001251910.
Figures


References
-
- Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Medical Care. 2001;39:II-46–II-54. - PubMed
-
- Buchan H, Sewell JR, Sweet M. Adopting Best Evidence in Practice:Translating evidence into practice. Med J Australia. 2004;180(Suppl 6):s43–s44. - PubMed
-
- Westfall JM, Mold J, Fagnan L. Practice-Based Research-“Blue Highways” on the NIH Roadmap. J Am Med Assoc. 2007;180(Suppl 6):s43–s44. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

