Interplacental uterine expression of genes involved in prostaglandin synthesis during canine pregnancy and at induced prepartum luteolysis/abortion
- PMID: 24884887
- PMCID: PMC4055803
- DOI: 10.1186/1477-7827-12-46
Interplacental uterine expression of genes involved in prostaglandin synthesis during canine pregnancy and at induced prepartum luteolysis/abortion
Abstract
Background: In the non-pregnant dog, ovarian cyclicity is independent of a uterine luteolysin. This is in contrast to pregnant animals where a prepartum increase of luteolytic PGF2α occurs, apparently originating in the pregnant uterus. Recently, the placenta as a source of prepartum prostaglandins (PGs) was investigated, indicating fetal trophoblast cells as the likely main source. However, the possible contribution of uterine interplacental tissues to the production of these hormones has not yet been thoroughly examined in the dog.
Methods: Several key factors involved in the production and/or actions of PGs were studied: cyclooxygenase 2 (COX2, PTGS2), PGF2α-synthase (PGFS/AKR1C3), PGE2-synthase (PGES), and the respective receptors FP (PTGFR), EP2 (PTGER2) and EP4 (PGTER4), 15-hydroxyprostaglandin dehydrogenase (HPGD), PG-transporter (PGT, SLCO2A1) and progesterone receptor. Their expression and localization patterns were assessed by Real Time PCR and immunohistology in the interplacental uterine sites from pregnant dogs during the pre-implantation period (days 8-12), post-implantation (days 18-25), mid-gestation (days 35-40) and during antigestagen-induced luteolysis/abortion.
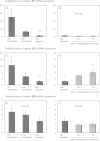
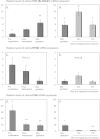
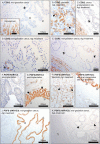
Results: Whereas only low COX2 expression was observed in uterine samples at all the selected time points, expression of PGFS/AKR1C3 strongly increased post-implantation. A gradual increase in PGES-mRNA expression was noted towards mid-gestation. FP-mRNA expression decreased significantly with the progression of pregnancy until mid-gestation. This was associated with clearly detectable expression of HPGD, which did not change significantly over time. The expression of FP and EP2-mRNA decreased significantly over time while EP4-mRNA expression remained unaffected. The antigestagen-treatment led to a significant increase in expression of COX2, PGES, EP2 and PGT (SLCO2A1) mRNA. COX2 was localized predominantly in the myometrium. The expression of PGFS/AKR1C3, which was unchanged, was localized mostly to the surface luminal epithelium. The expression of EP4, PGT and HPGH did not change during treatment, they were co-localized with PGES and EP2 in all uterine compartments.
Conclusions: The data clearly demonstrate the basic capability of the canine pregnant uterus to produce and respond to PGs and suggests their functions both as local regulatory factors involved in the establishment and maintenance of pregnancy, as well as potential contributors to the process of parturition, supporting the myometrial contractility associated with fetal expulsion.
Figures


Similar articles
-
Time related changes in luteal prostaglandin synthesis and steroidogenic capacity during pregnancy, normal and antiprogestin induced luteolysis in the bitch.Anim Reprod Sci. 2009 Nov;116(1-2):129-38. doi: 10.1016/j.anireprosci.2008.12.011. Epub 2008 Dec 24. Anim Reprod Sci. 2009. PMID: 19162415
-
Canine placenta: a source of prepartal prostaglandins during normal and antiprogestin-induced parturition.Reproduction. 2010 Mar;139(3):655-64. doi: 10.1530/REP-09-0140. Epub 2009 Nov 24. Reproduction. 2010. PMID: 19934344
-
Canine placental prostaglandin E2 synthase: expression, localization, and biological functions in providing substrates for prepartum PGF2alpha synthesis.Biol Reprod. 2014 Dec;91(6):154. doi: 10.1095/biolreprod.114.122929. Epub 2014 Oct 8. Biol Reprod. 2014. PMID: 25297547
-
Luteal regression vs. prepartum luteolysis: regulatory mechanisms governing canine corpus luteum function.Reprod Biol. 2014 Apr;14(2):89-102. doi: 10.1016/j.repbio.2013.11.004. Epub 2013 Dec 21. Reprod Biol. 2014. PMID: 24856467 Review.
-
Biochemistry of myometrial contractility.Baillieres Clin Obstet Gynaecol. 1992 Dec;6(4):755-69. doi: 10.1016/s0950-3552(05)80187-7. Baillieres Clin Obstet Gynaecol. 1992. PMID: 1335853 Review.
Cited by
-
Do uterine PTGS2, PGFS, and PTGFR expression play a role in canine uterine inertia?Cell Tissue Res. 2021 Jul;385(1):251-264. doi: 10.1007/s00441-021-03427-6. Epub 2021 Apr 8. Cell Tissue Res. 2021. PMID: 33830296 Free PMC article.
-
Insights into the role of PGF2α in canine periparturient myometrium.Front Physiol. 2024 May 28;15:1392080. doi: 10.3389/fphys.2024.1392080. eCollection 2024. Front Physiol. 2024. PMID: 38863475 Free PMC article.
-
Roles of Organic Anion Transporting Polypeptide 2A1 (OATP2A1/SLCO2A1) in Regulating the Pathophysiological Actions of Prostaglandins.AAPS J. 2017 Dec 4;20(1):13. doi: 10.1208/s12248-017-0163-8. AAPS J. 2017. PMID: 29204966 Review.
-
Analysis of Gene Expression Signatures in Cancer-Associated Stroma from Canine Mammary Tumours Reveals Molecular Homology to Human Breast Carcinomas.Int J Mol Sci. 2017 May 20;18(5):1101. doi: 10.3390/ijms18051101. Int J Mol Sci. 2017. PMID: 28531107 Free PMC article.
-
Hypoxia-inducible factor (HIF1alpha) inhibition modulates cumulus cell function and affects bovine oocyte maturation in vitro†.Biol Reprod. 2021 Feb 11;104(2):479-491. doi: 10.1093/biolre/ioaa196. Biol Reprod. 2021. PMID: 33095229 Free PMC article.
References
-
- Kowalewski MP. Endocrine and molecular control of luteal and placental function in dogs: a review. Reprod Domest Anim. 2012;47(Suppl 6):19–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

