The neural mobilization technique modulates the expression of endogenous opioids in the periaqueductal gray and improves muscle strength and mobility in rats with neuropathic pain
- PMID: 24884961
- PMCID: PMC4050394
- DOI: 10.1186/1744-9081-10-19
The neural mobilization technique modulates the expression of endogenous opioids in the periaqueductal gray and improves muscle strength and mobility in rats with neuropathic pain
Abstract
Background: The neural mobilization (NM) technique is a noninvasive method that has been proven to be clinically effective in reducing pain; however, the molecular mechanisms involved remain poorly understood. The aim of this study was to analyze whether NM alters the expression of the mu-opioid receptor (MOR), the delta-opioid receptor (DOR) and the Kappa-opioid receptor (KOR) in the periaqueductal gray (PAG) and improves locomotion and muscle force after chronic constriction injury (CCI) in rats.
Methods: The CCI was imposed on adult male rats followed by 10 sessions of NM every other day, starting 14 days after the CCI injury. At the end of the sessions, the PAG was analyzed using Western blot assays for opioid receptors. Locomotion was analyzed by the Sciatic functional index (SFI), and muscle force was analyzed by the BIOPAC system.
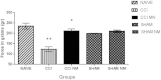
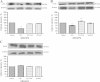
Results: An improvement in locomotion was observed in animals treated with NM compared with injured animals. Animals treated with NM showed an increase in maximal tetanic force of the tibialis anterior muscle of 172% (p < 0.001) compared with the CCI group. We also observed a decrease of 53% (p < 0.001) and 23% (p < 0.05) in DOR and KOR levels, respectively, after CCI injury compared to those from naive animals and an increase of 17% (p < 0.05) in KOR expression only after NM treatment compared to naive animals. There were no significant changes in MOR expression in the PAG.
Conclusion: These data provide evidence that a non-pharmacological NM technique facilitates pain relief by endogenous analgesic modulation.
Figures

Similar articles
-
Non-pharmacological treatment affects neuropeptide expression in neuropathic pain model.Brain Res. 2018 May 15;1687:60-65. doi: 10.1016/j.brainres.2018.02.034. Epub 2018 Feb 26. Brain Res. 2018. PMID: 29496478
-
Neurochemical effects of motor cortex stimulation in the periaqueductal gray during neuropathic pain.J Neurosurg. 2020 Jan 1;132(1):239-251. doi: 10.3171/2018.7.JNS173239. Epub 2019 Jan 4. J Neurosurg. 2020. PMID: 30611141
-
Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain.J Neurosci. 2018 May 16;38(20):4829-4839. doi: 10.1523/JNEUROSCI.2731-17.2018. Epub 2018 Apr 25. J Neurosci. 2018. PMID: 29695413 Free PMC article.
-
Treatment with steroid hormones and morphine alters general activity, sexual behavior, and opioid gene expression in female rats.Life Sci. 2014 May 28;104(1-2):47-54. doi: 10.1016/j.lfs.2014.03.021. Epub 2014 Mar 31. Life Sci. 2014. PMID: 24699004
-
Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans.Neuroimage. 2017 Feb 1;146:833-842. doi: 10.1016/j.neuroimage.2016.08.038. Epub 2016 Aug 21. Neuroimage. 2017. PMID: 27554530 Free PMC article.
Cited by
-
Preventive effect of the neurodynamic mobilization technique on delayed onset of muscle soreness: a randomized, single-blinded, placebo-controlled study.BMC Musculoskelet Disord. 2025 May 10;26(1):464. doi: 10.1186/s12891-025-08723-8. BMC Musculoskelet Disord. 2025. PMID: 40349018 Free PMC article. Clinical Trial.
-
Effects of Neural Mobilization on Sensory Dysfunction and Peripheral Nerve Degeneration in Rats With Painful Diabetic Neuropathy.Phys Ther. 2022 Oct 6;102(10):pzac104. doi: 10.1093/ptj/pzac104. Phys Ther. 2022. PMID: 35913760 Free PMC article.
-
Does the efficacy of neurodynamic treatments depend on the presence and type of criteria used to define neural mechanosensitivity in spinally-referred leg pain? A systematic review and meta-analysis.S Afr J Physiother. 2022 Jul 22;78(1):1627. doi: 10.4102/sajp.v78i1.1627. eCollection 2022. S Afr J Physiother. 2022. PMID: 35937092 Free PMC article. Review.
-
Peripheral and central changes induced by neural mobilization in animal models of neuropathic pain: a systematic review.Front Neurol. 2024 Jan 5;14:1289361. doi: 10.3389/fneur.2023.1289361. eCollection 2023. Front Neurol. 2024. PMID: 38249743 Free PMC article.
-
Effects of joint and nerve mobilisation on neuroimmune responses in animals and humans with neuromusculoskeletal conditions: a systematic review and meta-analysis.Pain Rep. 2021 Jun 3;6(2):e927. doi: 10.1097/PR9.0000000000000927. eCollection 2021 Jul-Aug. Pain Rep. 2021. PMID: 34104836 Free PMC article. Review.
References
-
- Devor M, Seltzer Z. In: Textbook of Pain. Melzack R, Wall PD, editor. London: Churchill Livingstone; 1999. Pathophysiology of damaged nerves in relation to chronic pain; pp. 129–164.
-
- Schaible HG. In: Handbook of Experimental Pharmacology. Stain C, editor. Jena, Germany: Springer-Verlag; 2007. Peripheral and central mechanisms of pain generation; pp. 3–28. (177). - PubMed
-
- Severo A, Calieron LG, Kuhn A. Compressão do nervo fibular comum por osteocondroma: relato de caso. Rev Bras Ortop. 2001;10(9):50–54.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

