Quantitative analysis of castration resistant prostate cancer progression through phosphoproteome signaling
- PMID: 24885093
- PMCID: PMC4031492
- DOI: 10.1186/1471-2407-14-325
Quantitative analysis of castration resistant prostate cancer progression through phosphoproteome signaling
Abstract
Background: Although recent progress has been made in treating castration resistant prostate cancer, the interplay of signaling pathways which enable castration resistant growth is incompletely understood. A data driven, multivariate approach, was used in this study to predict prostate cancer cell survival based on the phosphorylation levels of key proteins in PC3, LNCaP, and MDA-PCa-2b cell lines in response to EGF, IGF1, IL6, TNFα, dihydrotestosterone, and docetaxel treatment.
Methods: The prostate cancer cell lines were treated with ligands or inhibitors, cell lyates were collected, and the amount of phosphoprotein quantified using 384 well ELISA assays. In separate experiments, relative cell viability was determined using an MTT assay. Normalized data was imported into Matlab where regression analysis was performed.
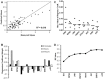
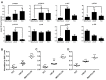
Results: Based on a linear model developed using partial least squares regression, p-Erk1/2 was found to correlate with castration resistant survival along with p-RPS6, and this model was determined to have a leave-one-out cross validated R2 value of 0.61. The effect of androgen on the phosphoproteome was examined, and increases in PI3K related phosphoproteins (p-Akt, p-RPS6, and p-GSK3) were observed which accounted for the majority of the significant increase in androgen-mediated cell survival. Simultaneous inhibition of the PI3K pathway and treatment with androgen resulted in a non-significant increase in survival. Given the strong effect of PI3K related signaling in enabling castration resistant survival, the specific effect of mTor versus complete inhibition was examined using targeted inhibitors. It was determine that mTor inhibition accounts for 52% of the effect of complete PI3K inhibition on cell survival. The differences in signaling between the cell lines were explored it was observed that MDA-PCa-2b exhibited far less activation of p-Erk in response to varying treatments, explaining one of the reasons for the lack of castration resistance.
Conclusion: In this work, regression analysis to the phosphoproteome was used to illustrate the sources of castration resistance between the cell lines including reduced p-Erk signaling in MDA-PCa-2b and variations in p-JNK across the cell lines, as well as studying the signaling pathways which androgen acts through, and determining the response to treatment with targeted inhibitors.
Figures





Similar articles
-
Combined AKT and MEK Pathway Blockade in Pre-Clinical Models of Enzalutamide-Resistant Prostate Cancer.PLoS One. 2016 Apr 5;11(4):e0152861. doi: 10.1371/journal.pone.0152861. eCollection 2016. PLoS One. 2016. PMID: 27046225 Free PMC article.
-
Reciprocal feedback inhibition of the androgen receptor and PI3K as a novel therapy for castrate-sensitive and -resistant prostate cancer.Oncotarget. 2015 Dec 8;6(39):41976-87. doi: 10.18632/oncotarget.5659. Oncotarget. 2015. PMID: 26506516 Free PMC article.
-
Analysis of cabazitaxel-resistant mechanism in human castration-resistant prostate cancer.Cancer Sci. 2018 Sep;109(9):2937-2945. doi: 10.1111/cas.13729. Epub 2018 Aug 6. Cancer Sci. 2018. PMID: 29989268 Free PMC article.
-
Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer.Endocr Relat Cancer. 2013 May 20;20(3):R83-99. doi: 10.1530/ERC-12-0394. Print 2013 Jun. Endocr Relat Cancer. 2013. PMID: 23456430 Review.
-
PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance.Asian J Androl. 2014 May-Jun;16(3):378-86. doi: 10.4103/1008-682X.122876. Asian J Androl. 2014. PMID: 24759575 Free PMC article. Review.
Cited by
-
Integrative proteomic and phosphoproteomic profiling of prostate cell lines.PLoS One. 2019 Nov 1;14(11):e0224148. doi: 10.1371/journal.pone.0224148. eCollection 2019. PLoS One. 2019. PMID: 31675377 Free PMC article.
-
Drug Repurposing of Pantoprazole and Vitamin C Targeting Tumor Microenvironment Conditions Improves Anticancer Effect in Metastatic Castration-Resistant Prostate Cancer.Front Oncol. 2021 Jul 7;11:660320. doi: 10.3389/fonc.2021.660320. eCollection 2021. Front Oncol. 2021. PMID: 34307134 Free PMC article.
-
Network analysis of an in vitro model of androgen-resistance in prostate cancer.BMC Cancer. 2015 Nov 10;15:883. doi: 10.1186/s12885-015-1884-7. BMC Cancer. 2015. PMID: 26553226 Free PMC article.
-
Logic Modeling in Quantitative Systems Pharmacology.CPT Pharmacometrics Syst Pharmacol. 2017 Aug;6(8):499-511. doi: 10.1002/psp4.12225. Epub 2017 Jul 29. CPT Pharmacometrics Syst Pharmacol. 2017. PMID: 28681552 Free PMC article.
References
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER cancer statistics review. http://seer.cancer.gov/csr/1975_2011/
-
- De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KM, Jones RL, Goodman OB, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scolz M, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. - DOI - PMC - PubMed
-
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL. Antitumor activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

