A monitoring and feedback tool embedded in a counselling protocol to increase physical activity of patients with COPD or type 2 diabetes in primary care: study protocol of a three-arm cluster randomised controlled trial
- PMID: 24885096
- PMCID: PMC4030038
- DOI: 10.1186/1471-2296-15-93
A monitoring and feedback tool embedded in a counselling protocol to increase physical activity of patients with COPD or type 2 diabetes in primary care: study protocol of a three-arm cluster randomised controlled trial
Abstract
Background: Physical activity is important for a healthy lifestyle. Although physical activity can delay complications and decrease the burden of the disease, the level of activity of patients with chronic obstructive pulmonary disease (COPD) or type 2 Diabetes Mellitus (DM2) is often far from optimal. To stimulate physical activity, a monitoring and feedback tool, consisting of an accelerometer linked to a smart phone and webserver (It's LiFe! tool), and a counselling protocol for practice nurses in primary care was developed (the Self-management Support Program). The main objective of this study is to measure the longitudinal effects of this counselling protocol and the added value of using the tool.
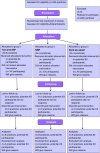
Methods/design: This three-armed cluster randomised controlled trial with 120 participants with COPD and 120 participants with DM2 (aged 40-70), compares the counselling protocol with and without the use of the tool (group 1 and 2) with usual care (group 3). Recruitment takes place at GP practices in the southern regions of the Netherlands. Randomisation takes place at the practice level. The intended sample (three arms of 8 practices) powers the study to detect a 10-minute difference of moderate and intense physical activity per day between groups 1 and 3. Participants in the intervention groups have to visit the practice nurse 3-4 times for physical activity counselling, in a 4-6-month period. Specific activity goals tailored to the individual patient's preferences and needs will be set. In addition, participants in group 1 will be instructed to use the tool in daily life. The primary outcome, physical activity, will be measured in all groups with a physical activity monitor (PAM). Secondary outcomes are quality of life, general - and exercise - self-efficacy, and health status. Follow-up will take place after 6 and 9 months. Separately, a process evaluation will be conducted to explore reasons for trial non-participation, and the intervention's acceptability for participating patients and nurses.
Discussion: Results of this study will give insight into the effects of the It's LiFe! monitoring and feedback tool combined with care from a practice nurse for people with COPD or DM2 on physical activity.
Trial registration: ClinicalTrials.gov: NCT01867970.
Figures
References
-
- World Health O. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva, Switzerland: World Health Organization; 2009.
-
- Global recommendations on physical activity for health. http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/ - PubMed
-
- Nederlandse Diabetes F. NDF Zorgstandaard: transparantie en kwaliteit van diabeteszorg voor mensen met diabetes type 2. Amersfoort: Nederlandse Diabetes Federatie; 2007.
-
- Long Alliantie N. Zorgstandaard COPD. Amersfoort: Long Alliantie Nederland [Host]; 2010.
-
- Heijmans M, Spreeuwenberg P, Rijken M. Ontwikkelingen in de zorg voor chronisch zieken Rapportage 2010 In. NIVEL: Utrecht; 2010.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous