Design and implementation of a high yield production system for recombinant expression of peptides
- PMID: 24885242
- PMCID: PMC4022411
- DOI: 10.1186/1475-2859-13-65
Design and implementation of a high yield production system for recombinant expression of peptides
Abstract
Background: Making peptide pharmaceuticals involves challenging processes where many barriers, which include production and manufacture, need to be overcome. A non common but interesting research area is related to peptides with intracellular targets, which opens up new possibilities, allowing the modulation of processes occurring within the cell or interference with signaling pathways. However, if the bioactive sequence requires fusion to a carrier peptide to allow access into the cell, the resulting peptide could be such a length that traditional production could be difficult. The goal of the present study was the development of a flexible recombinant expression and purification system for peptides, as a contribution to the discovery and development of these potentially new drugs.
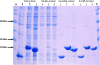
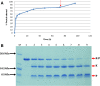
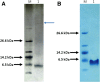
Results: In this work, a high throughput recombinant expression and purification system for production of cell penetrating peptides in Escherichia coli has been designed and implemented. The system designed produces target peptides in an insoluble form by fusion to a hexahistidine tagged ketosteroid isomerase which is then separated by a highly efficient thrombin cleavage reaction procedure. The expression system was tested on the anticancer peptides p53pAnt and PNC27. These peptides comprise the C-terminal region and the N-terminal region of the protein p53, respectively, fused by its carboxyl terminal extreme to the cell penetrating peptide Penetratin. High yields of purified recombinant fused peptides were obtained in both cases; nevertheless, thrombin cleavage reaction was successful only for p53pAnt peptide release. The features of the system, together with the procedure developed, allow achievement of high production yields of over 30 mg of highly pure p53pAnt peptide per g of dry cell mass. It is proposed that the system could be used for production of other peptides at a similar yield.
Conclusions: This study provides a system suitable for recombinant production of peptides for scientific research, including biological assays.
Figures

Similar articles
-
Chemical Cleavage of an Asp-Cys Sequence Allows Efficient Production of Recombinant Peptides with an N-Terminal Cysteine Residue.Bioconjug Chem. 2018 Apr 18;29(4):1373-1383. doi: 10.1021/acs.bioconjchem.8b00083. Epub 2018 Mar 21. Bioconjug Chem. 2018. PMID: 29528625
-
Production of cell-penetrating peptides in Escherichia coli using an intein-mediated system.Appl Biochem Biotechnol. 2015 Mar;175(6):3025-37. doi: 10.1007/s12010-015-1484-7. Epub 2015 Jan 14. Appl Biochem Biotechnol. 2015. PMID: 25586490
-
Facilitation of expression and purification of an antimicrobial peptide by fusion with baculoviral polyhedrin in Escherichia coli.Appl Environ Microbiol. 2005 Sep;71(9):5038-43. doi: 10.1128/AEM.71.9.5038-5043.2005. Appl Environ Microbiol. 2005. PMID: 16151084 Free PMC article.
-
Targeted expression, purification, and cleavage of fusion proteins from inclusion bodies in Escherichia coli.FEBS Lett. 2014 Jan 21;588(2):247-52. doi: 10.1016/j.febslet.2013.09.028. Epub 2013 Sep 27. FEBS Lett. 2014. PMID: 24076468 Review.
-
Advances in recombinant protein production in microorganisms and functional peptide tags.Biosci Biotechnol Biochem. 2024 Dec 23;89(1):1-10. doi: 10.1093/bbb/zbae147. Biosci Biotechnol Biochem. 2024. PMID: 39479788 Review.
Cited by
-
Strategies for the Production of Soluble Interferon-Alpha Consensus and Potential Application in Arboviruses and SARS-CoV-2.Life (Basel). 2021 May 21;11(6):460. doi: 10.3390/life11060460. Life (Basel). 2021. PMID: 34063766 Free PMC article.
-
Engineered Akkermansia muciniphila: A promising agent against diseases (Review).Exp Ther Med. 2020 Dec;20(6):285. doi: 10.3892/etm.2020.9415. Epub 2020 Oct 29. Exp Ther Med. 2020. PMID: 33209129 Free PMC article. Review.
-
Antioxidant Peptides Derived from Woody Oil Resources: Mechanisms of Redox Protection and Emerging Therapeutic Opportunities.Pharmaceuticals (Basel). 2025 Jun 4;18(6):842. doi: 10.3390/ph18060842. Pharmaceuticals (Basel). 2025. PMID: 40573238 Free PMC article. Review.
-
Cell-penetrating peptides for sustainable agriculture.Trends Plant Sci. 2024 Oct;29(10):1131-1144. doi: 10.1016/j.tplants.2024.05.011. Epub 2024 Jun 19. Trends Plant Sci. 2024. PMID: 38902122 Review.
-
Efficient recombinant production of mouse-derived cryptdin family peptides by a novel facilitation strategy for inclusion body formation.Microb Cell Fact. 2023 Jan 13;22(1):9. doi: 10.1186/s12934-023-02016-2. Microb Cell Fact. 2023. PMID: 36635697 Free PMC article.
References
-
- Guzman F, Barberis S, Illanes A. Electronic Journal of Biotechnology, vol. 10. Valparaiso: Pontificia Universidad Católica de Valparaíso; 2007. Peptide synthesis: chemical or enzymatic.
-
- Lee EJ, Kim HS, Lee EY. Recombinant biocatalytic and cell-free synthesis of HIV fusion inhibitor. J Ind Eng Chem. 2005;11:515–521.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

