Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus
- PMID: 24885320
- PMCID: PMC4018502
- DOI: 10.1186/1743-422X-11-82
Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus
Abstract
Background: Public health risks associated to infection by human coronaviruses remain considerable and vaccination is a key option for preventing the resurgence of severe acute respiratory syndrome coronavirus (SARS-CoV). We have previously reported that antibodies elicited by a SARS-CoV vaccine candidate based on recombinant, full-length SARS-CoV Spike-protein trimers, trigger infection of immune cell lines. These observations prompted us to investigate the molecular mechanisms and responses to antibody-mediated infection in human macrophages.
Methods: We have used primary human immune cells to evaluate their susceptibility to infection by SARS-CoV in the presence of anti-Spike antibodies. Fluorescence microscopy and real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) were utilized to assess occurrence and consequences of infection. To gain insight into the underlying molecular mechanism, we performed mutational analysis with a series of truncated and chimeric constructs of fragment crystallizable γ receptors (FcγR), which bind antibody-coated pathogens.
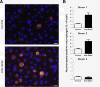
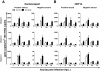
Results: We show here that anti-Spike immune serum increased infection of human monocyte-derived macrophages by replication-competent SARS-CoV as well as Spike-pseudotyped lentiviral particles (SARS-CoVpp). Macrophages infected with SARS-CoV, however, did not support productive replication of the virus. Purified anti-viral IgGs, but not other soluble factor(s) from heat-inactivated mouse immune serum, were sufficient to enhance infection. Antibody-mediated infection was dependent on signaling-competent members of the human FcγRII family, which were shown to confer susceptibility to otherwise naïve ST486 cells, as binding of immune complexes to cell surface FcγRII was necessary but not sufficient to trigger antibody-dependent enhancement (ADE) of infection. Furthermore, only FcγRII with intact cytoplasmic signaling domains were competent to sustain ADE of SARS-CoVpp infection, thus providing additional information on the role of downstream signaling by FcγRII.
Conclusions: These results demonstrate that human macrophages can be infected by SARS-CoV as a result of IgG-mediated ADE and indicate that this infection route requires signaling pathways activated downstream of binding to FcγRII receptors.
Figures


Similar articles
-
Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway.J Virol. 2011 Oct;85(20):10582-97. doi: 10.1128/JVI.00671-11. Epub 2011 Jul 20. J Virol. 2011. PMID: 21775467 Free PMC article.
-
Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins.Biochem Biophys Res Commun. 2014 Aug 22;451(2):208-14. doi: 10.1016/j.bbrc.2014.07.090. Epub 2014 Jul 26. Biochem Biophys Res Commun. 2014. PMID: 25073113 Free PMC article.
-
Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro.Vaccine. 2007 Jan 8;25(4):729-40. doi: 10.1016/j.vaccine.2006.08.011. Epub 2006 Aug 22. Vaccine. 2007. PMID: 17049691 Free PMC article.
-
A Review: Understanding Molecular Mechanisms of Antibody-Dependent Enhancement in Viral Infections.Vaccines (Basel). 2023 Jul 14;11(7):1240. doi: 10.3390/vaccines11071240. Vaccines (Basel). 2023. PMID: 37515055 Free PMC article. Review.
-
Antibody Dependent Enhancement of SARS-CoV-2 Infection in the Era of Rapid Vaccine Development.Med Arch. 2022 Oct;76(5):383-386. doi: 10.5455/medarh.2022.76.383-386. Med Arch. 2022. PMID: 36545460 Free PMC article. Review.
Cited by
-
SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction.J Virol. 2021 Apr 26;95(10):e00110-21. doi: 10.1128/JVI.00110-21. Epub 2021 Feb 26. J Virol. 2021. PMID: 33637603 Free PMC article.
-
COVID-19: Immunology, Immunopathogenesis and Potential Therapies.Int Rev Immunol. 2022;41(2):171-206. doi: 10.1080/08830185.2021.1883600. Epub 2021 Feb 27. Int Rev Immunol. 2022. PMID: 33641587 Free PMC article. Review.
-
Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development.Front Microbiol. 2022 Aug 10;13:932408. doi: 10.3389/fmicb.2022.932408. eCollection 2022. Front Microbiol. 2022. PMID: 36033843 Free PMC article. Review.
-
Ross River Virus Immune Evasion Strategies and the Relevance to Post-viral Fatigue, and Myalgic Encephalomyelitis Onset.Front Med (Lausanne). 2021 Mar 24;8:662513. doi: 10.3389/fmed.2021.662513. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33842517 Free PMC article.
-
The role of IgG Fc receptors in antibody-dependent enhancement.Nat Rev Immunol. 2020 Oct;20(10):633-643. doi: 10.1038/s41577-020-00410-0. Epub 2020 Aug 11. Nat Rev Immunol. 2020. PMID: 32782358 Free PMC article. Review.
References
-
- Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, Uyeki TM. Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza AV. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358:261–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

