Demethylation of the miR-146a promoter by 5-Aza-2'-deoxycytidine correlates with delayed progression of castration-resistant prostate cancer
- PMID: 24885368
- PMCID: PMC4024097
- DOI: 10.1186/1471-2407-14-308
Demethylation of the miR-146a promoter by 5-Aza-2'-deoxycytidine correlates with delayed progression of castration-resistant prostate cancer
Abstract
Background: Androgen deprivation therapy is the primary strategy for the treatment of advanced prostate cancer; however, after an initial regression, most patients will inevitably develop a fatal androgen-independent tumor. Therefore, understanding the mechanisms of the transition to androgen independence prostate cancer is critical to identify new ways to treat older patients who are ineligible for conventional chemotherapy.
Methods: The effects of 5-Aza-2'-deoxycytidine (5-Aza-CdR) on the viability and the apoptosis of the androgen-dependent (LNCaP) and androgen-independent (PC3) cell lines were examined by MTS assay and western blot analysis for the activation of caspase-3. The subcutaneous LNCaP xenografts were established in a nude mice model. MiR-146a and DNMTs expressions were analyzed by qRT-PCR and DNA methylation rates of LINE-1 were measured by COBRA-IRS to determine the global DNA methylation levels. The methylation levels of miR-146a promoter region in the different groups were quantified by the bisulfite sequencing PCR (BSP) assay.
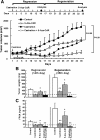
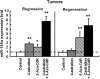
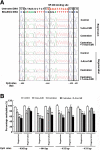
Results: We validated that 5-Aza-CdR induced cell death and increased miR-146a expression in both LNCaP and PC3 cells. Notably, the expression of miR-146a in LNCaP cells was much higher than in PC3 cells. MiR-146a inhibitor was shown to suppress apoptosis in 5-Aza-CdR-treated cells. In a castrate mouse LNCaP xenograft model, 5-Aza-CdR significantly suppressed the tumors growth and also inhibited prostate cancer progression. Meanwhile, miR-146a expression was significantly enhanced in the tumor xenografts of 5-Aza-CdR-treated mice and the androgen-dependent but not the androgen-independent stage of castrated mice. In particular, the expression of miR-146a was significantly augmented in both stages of the combined treatment (castration and 5-Aza-CdR). Additionally, the methylation percentage of the two CpG sites (-444 bp and -433 bp), which were around the NF-κB binding site at miR-146a promoter, showed the lowest methylation levels among all CpG sites in the combined treatment tumors of both stages.
Conclusion: Up-regulating miR-146a expression via the hypomethylation of the miR-146a promoter by 5-Aza-CdR was correlated with delayed progression of castration-resistant prostate cancers. Moreover, site-specific DNA methylation may play an important role in miR-146a expression in androgen-dependent prostate cancer progression to androgen-independent prostate cancer and therefore provides a potentially useful biomarker for assessing drug efficacy in prostate cancer.
Figures


References
-
- Goldenberg SL, Gleave ME, Taylor D, Bruchovsky N. Clinical experience with intermittent androgen suppression in prostate cancer: minimum of 3 Years’ follow-Up. Mol Urol. 1999;3(3):287–292. - PubMed
-
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–428. - PubMed
-
- Haldrup C, Mundbjerg K, Vestergaard EM, Lamy P, Wild P, Schulz WA, Arsov C, Visakorpi T, Borre M, Hoyer S, Orntoft TF, Sørensen KD. DNA methylation signatures for prediction of biochemical recurrence after radical prostatectomy of clinically localized prostate cancer. J Clin Oncol. 2013;31(26):3250–3258. doi: 10.1200/JCO.2012.47.1847. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

