Starvation of low-density lipoprotein-derived cholesterol induces bradyzoite conversion in Toxoplasma gondii
- PMID: 24885547
- PMCID: PMC4046157
- DOI: 10.1186/1756-3305-7-248
Starvation of low-density lipoprotein-derived cholesterol induces bradyzoite conversion in Toxoplasma gondii
Abstract
Background: Lacking enzymes for sterol synthesis, the intracellular protozoan Toxoplasma gondii scavenges cholesterol from host cells to multiply. T. gondii has a complex life cycle consisting of two asexual stages; the proliferative stage (tachyzoite), and the latent stage characterized by tissue cysts (bradyzoite). In vitro, bradyzoite development can be induced by mimicking host immune response stressors through treatment with IFN-γ, heat shock, nitric oxide, and high pH. However, the extent to which host nutrients contribute to stage conversion in T. gondii is unknown. In this study, we examined the impact of host cholesterol levels on stage conversion in this parasite.
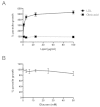
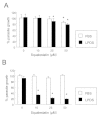
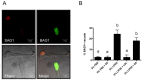
Methods: Growth of T. gondii tachyzoites (ME49 strain) was investigated in Chinese hamster ovary (CHO) cells using various concentrations of low-density lipoprotein (LDL), oleic acid, or glucose. Squalestatin, which is an inhibitor of squalene synthase and is, therefore, an inhibitor of sterol synthesis, was used to treat the CHO cells. Tachyzoite to bradyzoite conversion rates were analyzed by indirect fluorescent antibody tests.
Results: Parasite growth was significantly enhanced by addition of exogenous LDL, whereas no such enhancement occurred with oleic acids or glucose. In ME49, growth inhibition from squalestatin treatment was not obvious. Although growth of the RH strain was unaffected by squalestatin in the presence of lipoprotein, in its absence growth of this strain was suppressed. The frequency of BAG1-positive vacuoles in ME49 increased under lipoprotein-free conditions. However, addition of exogenous LDL did not increase tachyzoite to bradyzoite conversion in this strain. Furthermore, treatment with squalestatin did not enhance stage conversion.
Conclusion: Our results suggest that LDL-derived cholesterol levels play a crucial role in bradyzoite conversion in T. gondii.
Figures
Similar articles
-
Real-time RT-PCR on SAG1 and BAG1 gene expression during stage conversion in immunosuppressed mice infected with Toxoplasma gondii Tehran strain.Korean J Parasitol. 2012 Sep;50(3):199-205. doi: 10.3347/kjp.2012.50.3.199. Epub 2012 Aug 13. Korean J Parasitol. 2012. PMID: 22949746 Free PMC article.
-
Toxoplasma gondii bradyzoite-specific BAG1 is nonessential for cyst formation due to compensation by other heat-shock proteins.Parasit Vectors. 2024 Jul 30;17(1):322. doi: 10.1186/s13071-024-06339-w. Parasit Vectors. 2024. PMID: 39080770 Free PMC article.
-
Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host.Int J Parasitol. 2004 Mar 9;34(3):347-60. doi: 10.1016/j.ijpara.2003.11.024. Int J Parasitol. 2004. PMID: 15003495
-
Stress-related and spontaneous stage differentiation of Toxoplasma gondii.Mol Biosyst. 2008 Aug;4(8):824-34. doi: 10.1039/b800520f. Epub 2008 Jun 2. Mol Biosyst. 2008. PMID: 18633484 Review.
-
Toxoplasma gondii: determinants of tachyzoite to bradyzoite conversion.Parasitol Res. 2010 Jul;107(2):253-60. doi: 10.1007/s00436-010-1899-6. Epub 2010 Jun 1. Parasitol Res. 2010. PMID: 20514494 Free PMC article. Review.
Cited by
-
A Novel Nuclear Protein Complex Controlling the Expression of Developmentally Regulated Genes in Toxoplasma Gondii.Adv Sci (Weinh). 2025 Feb;12(7):e2412000. doi: 10.1002/advs.202412000. Epub 2024 Dec 24. Adv Sci (Weinh). 2025. PMID: 39716984 Free PMC article.
-
Evaluation of the Protective Effect of Deoxyribonucleic Acid Vaccines Encoding Granule Antigen 2 and 5 Against Acute Toxoplasmosis in BALB/c Mice.Am J Trop Med Hyg. 2017 Jun;96(6):1441-1447. doi: 10.4269/ajtmh.16-0548. Am J Trop Med Hyg. 2017. PMID: 28719288 Free PMC article.
-
Constitutive upregulation of transcription factors underlies permissive bradyzoite differentiation in a natural isolate of Toxoplasma gondii.mBio. 2024 Sep 11;15(9):e0064124. doi: 10.1128/mbio.00641-24. Epub 2024 Aug 16. mBio. 2024. PMID: 39150246 Free PMC article.
-
Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Regulate Maturation of the Cyst Wall.mSphere. 2020 Jan 15;5(1):e00851-19. doi: 10.1128/mSphere.00851-19. mSphere. 2020. PMID: 31941814 Free PMC article.
-
Novel Approaches To Kill Toxoplasma gondii by Exploiting the Uncontrolled Uptake of Unsaturated Fatty Acids and Vulnerability to Lipid Storage Inhibition of the Parasite.Antimicrob Agents Chemother. 2018 Sep 24;62(10):e00347-18. doi: 10.1128/AAC.00347-18. Print 2018 Oct. Antimicrob Agents Chemother. 2018. PMID: 30061287 Free PMC article.
References
-
- Charron AJ, Sibley LD. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J Cell Sci. 2002;115:3049–3059. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

