BMP2-induced chemotaxis requires PI3K p55γ/p110α-dependent phosphatidylinositol (3,4,5)-triphosphate production and LL5β recruitment at the cytocortex
- PMID: 24885555
- PMCID: PMC4071339
- DOI: 10.1186/1741-7007-12-43
BMP2-induced chemotaxis requires PI3K p55γ/p110α-dependent phosphatidylinositol (3,4,5)-triphosphate production and LL5β recruitment at the cytocortex
Abstract
Background: BMP-induced chemotaxis of mesenchymal progenitors is fundamental for vertebrate development, disease and tissue repair. BMP2 induces Smad and non-Smad signalling. Whereas signal transduction via Smads lead to transcriptional responses, non-Smad signalling induces both, transcriptional and immediate/early non-transcriptional responses. However, the molecular mechanisms by which BMP2 facilitates planar cell polarity, cortical actin rearrangements, lamellipodia formation and chemotaxis of mesenchymal progenitors are poorly understood. Our aim was to uncover the molecular mechanism by which BMP2 facilitates chemotaxis via the BMP2-dependent activation of PI3K and spatiotemporal control of PIP3 production important for actin rearrangements at the mesenchymal cell cytocortex.
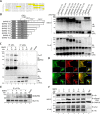
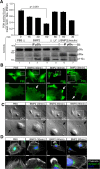
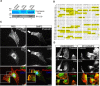
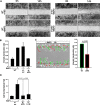
Results: We unveiled the molecular mechanism by which BMP2 induces non-Smad signalling by PI3K and the role of the second messenger PIP3 in BMP2-induced planar cell polarity, cortical actin reorganisation and lamellipodia formation. By using protein interaction studies, we identified the class Ia PI3K regulatory subunit p55γ to act as a specific and non-redundant binding partner for BMP receptor type II (BMPRII) in concert with the catalytic subunit p110α. We mapped the PI3K interaction to a region within the BMPRII kinase. Either BMP2 stimulation or increasing amounts of BMPRI facilitated p55γ association with BMPRII, but BMPRII kinase activity was not required for the interaction. We visualised BMP2-dependent PIP3 production via PI3K p55γ/p110α and were able to localise PIP3 to the leading edge of intact cells during the process of BMP2-induced planar cell polarity and actin dependent lamellipodia formation. Using mass spectrometry, we found the highly PIP3-sensitive PH-domain protein LL5β to act as a novel BMP2 effector in orchestrating cortical actin rearrangements. By use of live cell imaging we found that knock-down of p55γ or LL5β or pharmacological inhibition of PI3K impaired BMP2-induced migratory responses.
Conclusions: Our results provide evidence for an important contribution of the BMP2-PI3K (p55γ/p110α)- PIP3-LL5β signalling axis in mesenchymal progenitor cell chemotaxis. We demonstrate molecular insights into BMP2-induced PI3K signalling on the level of actin reorganisation at the leading edge cytocortex. These findings are important to better understand BMP2-induced cytoskeletal reorganisation and chemotaxis of mesenchymal progenitors in different physiological or pathophysiological contexts.
Figures




Similar articles
-
Nephrin regulates lamellipodia formation by assembling a protein complex that includes Ship2, filamin and lamellipodin.PLoS One. 2011;6(12):e28710. doi: 10.1371/journal.pone.0028710. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194892 Free PMC article.
-
Suppression of chemotaxis by SSeCKS via scaffolding of phosphoinositol phosphates and the recruitment of the Cdc42 GEF, Frabin, to the leading edge.PLoS One. 2014 Oct 30;9(10):e111534. doi: 10.1371/journal.pone.0111534. eCollection 2014. PLoS One. 2014. PMID: 25356636 Free PMC article.
-
Novel pathways of F-actin polymerization in the human neutrophil.Blood. 2003 Sep 15;102(6):2251-8. doi: 10.1182/blood-2002-09-2936. Epub 2003 May 22. Blood. 2003. PMID: 12763941
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Pathophysiology and therapeutic relevance of PI3K(p110α) protein in atrial fibrillation: A non-interventional molecular therapy strategy.Pharmacol Res. 2021 Mar;165:105415. doi: 10.1016/j.phrs.2020.105415. Epub 2021 Jan 4. Pharmacol Res. 2021. PMID: 33412279 Review.
Cited by
-
Array CGH Analysis of Paired Blood and Tumor Samples from Patients with Sporadic Wilms Tumor.PLoS One. 2015 Aug 28;10(8):e0136812. doi: 10.1371/journal.pone.0136812. eCollection 2015. PLoS One. 2015. PMID: 26317783 Free PMC article.
-
Bone morphogenetic protein receptor 2 inhibition destabilizes microtubules promoting the activation of lysosomes and cell death of lung cancer cells.Cell Commun Signal. 2021 Sep 25;19(1):97. doi: 10.1186/s12964-021-00743-w. Cell Commun Signal. 2021. PMID: 34563224 Free PMC article.
-
Role of bone morphogenetic proteins in sprouting angiogenesis: differential BMP receptor-dependent signaling pathways balance stalk vs. tip cell competence.FASEB J. 2017 Nov;31(11):4720-4733. doi: 10.1096/fj.201700193RR. Epub 2017 Jul 21. FASEB J. 2017. PMID: 28733457 Free PMC article.
-
VE-cadherin facilitates BMP-induced endothelial cell permeability and signaling.J Cell Sci. 2016 Jan 1;129(1):206-18. doi: 10.1242/jcs.179960. Epub 2015 Nov 23. J Cell Sci. 2016. PMID: 26598555 Free PMC article.
-
Dysregulation of angiogenesis-specific signalling in adult testis results in xenograft degeneration.Sci Rep. 2017 Jun 1;7(1):2605. doi: 10.1038/s41598-017-02604-4. Sci Rep. 2017. PMID: 28572601 Free PMC article.
References
-
- Ruschke K, Hiepen C, Becker J, Knaus P. BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res. 2012;12:521–544. - PubMed
-
- Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007;12:2493–2501. - PubMed
-
- Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;12:305–312. - PubMed
-
- Finkenzeller G, Hager S, Stark GB. Effects of bone morphogenetic protein 2 on human umbilical vein endothelial cells. Microvasc Res. 2012;12:81–85. - PubMed
-
- Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;12:1407–1412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

